21-Deoxycortisone
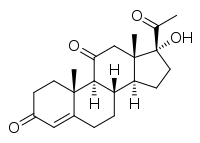
21-Deoxycortisone, also known as 21-desoxycortisone, 11-keto-17α-hydroxyprogesterone, or 17α-hydroxypregn-4-ene-3,11,20-trione, is a naturally occurring, endogenous steroid and minor intermediate and metabolite in corticosteroid metabolism. It is related to 21-deoxycortisol (11β,17α-dihydroxyprogesterone) and is reversibly formed from it by 11β-hydroxysteroid dehydrogenase, analogously to the reversible formation of cortisone from cortisol.[1] 21-Deoxycortisone can be transformed into cortisone by 21-hydroxylase.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(8S,9S,10R,13S,14S,17R)-17-Acetyl-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,11-dione | |
| Other names
21-Desoxycortisone; 11-Keto-17α-hydroxyprogesterone; 17α-Hydroxy-11-ketoprogesterone; 17α-Hydroxypregn-4-en-3,11,20-trione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.947 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H28O4 | |
| Molar mass | 344.451 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Homma K, Hasegawa T, Takeshita E, Watanabe K, Anzo M, Toyoura T, Jinno K, Ohashi T, Hamajima T, Takahashi Y, Takahashi T, Matsuo N (2004). "Elevated urine pregnanetriolone definitively establishes the diagnosis of classical 21-hydroxylase deficiency in term and preterm neonates". J. Clin. Endocrinol. Metab. 89 (12): 6087–91. doi:10.1210/jc.2004-0473. PMID 15579762.
- ROSENFELD G, UNGAR F, DORFMAN RI, PINCUS G (1955). "Irradiation and adrenal steroidogenesis: steroid transformations by irradiated isolated perfused calf adrenals". Endocrinology. 56 (1): 24–9. doi:10.1210/endo-56-1-24. PMID 13220521.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.