7β-Hydroxyepiandrosterone
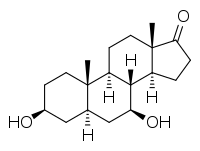
7β-Hydroxyepiandrosterone (7β-OH-EPIA), also known as 5α-androstan-3β,7β-diol-17-one, is an endogenous androgen, estrogen, and neurosteroid that is produced from dehydroepiandrosterone and epiandrosterone.[1][2][3] It has neuroprotective effects and, along with 7α-hydroxyepiandrosterone, may mediate the neuroprotective effects of DHEA.[1] 7β-OH-EPIA may act as a highly potent antagonist of the G protein-coupled estrogen receptor (GPER) (affinity of <1 nM).[4]
 | |
| Names | |
|---|---|
| IUPAC name
(3S,5R,7S,8R,9S,10S,13S,14S)-3,7-Dihydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,14,15,16-tetradecahydrocyclopenta[a]phenanthren-17-one | |
| Other names
7β-OH-EPIA; 5α-Androstan-3β,7β-diol-17-one; 3β,7β-Dihydroxy-5α-androstan-17-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C19H30O3 | |
| Molar mass | 306.446 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Dudas B, Hanin I, Rose M, Wülfert E (2004). "Protection against inflammatory neurodegeneration and glial cell death by 7beta-hydroxy epiandrosterone, a novel neurosteroid". Neurobiol. Dis. 15 (2): 262–8. doi:10.1016/j.nbd.2003.11.001. PMID 15006696. S2CID 24078411.
- Sandra N, Ester P, Marie-Agnès P, Robert M, Olivier H (2012). "The DHEA metabolite 7β-hydroxy-epiandrosterone exerts anti-estrogenic effects on breast cancer cell lines" (PDF). Steroids. 77 (5): 542–51. doi:10.1016/j.steroids.2012.01.019. PMID 22342541. S2CID 140140008.
- Miller KK, Al-Rayyan N, Ivanova MM, Mattingly KA, Ripp SL, Klinge CM, Prough RA (2013). "DHEA metabolites activate estrogen receptors alpha and beta". Steroids. 78 (1): 15–25. doi:10.1016/j.steroids.2012.10.002. PMC 3529809. PMID 23123738.
- Prossnitz ER, Arterburn JB (July 2015). "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacol. Rev. 67 (3): 505–40. doi:10.1124/pr.114.009712. PMC 4485017. PMID 26023144.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.