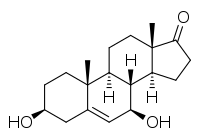
7β-Hydroxy-DHEA
7β-Hydroxydehydroepiandrosterone (7β-hydroxy-DHEA; 7β-OH-DHEA), also known as 3β,7β-dihydroxyandrost-4-ene-17-one, is an endogenous, naturally occurring steroid and a metabolite of dehydroepiandrosterone (DHEA). The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA.[1] 7β-OH-DHEA has weak antiestrogenic activity, selectively antagonizing the estrogen receptor ERβ.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(3S,7R,8R,9S,10R,13S,14S)-3,7-Dihydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one | |
| Other names
7β-OH-DHEA; 3β,7β-Dihydroxyandrost-4-ene-17-one; Androst-4-en-3β,7β-diol-17-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H28O3 | |
| Molar mass | 304.430 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
7β-OH-DHEA is on the World Anti-Doping Agency list of prohibited substances in sporting.[3]
References
- Li H, Liu HM, Ge W, Huang L, Shan L (2005). "Synthesis of 7alpha-hydroxy-dehydroepiandrosterone and 7beta-hydroxy-dehydroepiandrosterone". Steroids. 70 (14): 970–3. doi:10.1016/j.steroids.2005.07.006. PMID 16143359. S2CID 53294855.
he major metabolic pathway for DHEA in extra-hepatic tissues is via 7-hydroxylation [18], [19] and [20].
- Miller KK, Al-Rayyan N, Ivanova MM, Mattingly KA, Ripp SL, Klinge CM, Prough RA (2013). "DHEA metabolites activate estrogen receptors alpha and beta". Steroids. 78 (1): 15–25. doi:10.1016/j.steroids.2012.10.002. PMC 3529809. PMID 23123738.
- https://www.wada-ama.org/en/content/what-is-prohibited/prohibited-at-all-times/anabolic-agents
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.