Dehydroandrosterone
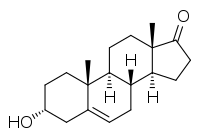
Dehydroandrosterone (DHA), or 5-dehydroandrosterone (5-DHA), also known as isoandrostenolone, as well as androst-5-en-3α-ol-17-one, is an endogenous androgen steroid hormone.[1][2] It is the 3α-epimer of dehydroepiandrosterone (DHEA; androst-5-en-3β-ol-17-one) and the 5(6)-dehydrogenated and non-5α-reduced analogue of androsterone (5α-androstan-3α-ol-17-one).[2] DHA is produced in and secreted from the adrenal glands, along with other weak androgens like DHEA, androstenediol, and androstenedione.[3]
 | |
| Names | |
|---|---|
| IUPAC name
(3R,8R,9S,10R,13S,14S)-3-Hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one | |
| Other names
DHA; 5-Dehydroandrosterone; 5-DHA; Androst-5-en-3α-ol-17-one; 3α-Hydroxyandrost-5-en-17-one; Isoandrostenolone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H28O2 | |
| Molar mass | 288.431 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
References
- http://www.hmdb.ca/metabolites/HMDB05962
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–. ISBN 978-1-4757-2085-3.
- Alfred E. Chang; Patricia A. Ganz; Daniel F. Hayes; Timothy Kinsella; Harvey I. Pass; Joan H. Schiller; Richard M. Stone; Victor Strecher (8 December 2007). Oncology: An Evidence-Based Approach. Springer Science & Business Media. pp. 75–. ISBN 978-0-387-31056-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
