Chlorovirus
Chlorovirus, also known as Chlorella virus, is a genus of giant double-stranded DNA viruses, in the family Phycodnaviridae. This genus is found globally in freshwater environments[1] where freshwater microscopic algae serve as natural hosts. There are currently 19 species in this genus including the type species Paramecium bursaria Chlorella virus 1.[2][3]
| Chlorovirus | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Varidnaviria |
| Kingdom: | Bamfordvirae |
| Phylum: | Nucleocytoviricota |
| Class: | Megaviricetes |
| Order: | Algavirales |
| Family: | Phycodnaviridae |
| Genus: | Chlorovirus |
| Type species | |
| Paramecium bursaria Chlorella virus 1 | |
Chlorovirus was initially discovered in 1981 by Russel H. Meints, James L. Van Etten, Daniel Kuczmarski, Kit Lee, and Barbara Ang while attempting to culture Chlorella-like algae. During the attempted process viral particles were discovered in the cells 2 to 6 hours after being initially isolated, followed by lysis after 12 to 20 hours. This virus was initially called HVCV (Hydra viridis Chlorella virus) since it was first found to infect Chlorella-like algae.[4][5]
Though relatively new to virologists and thus not extensively studied, one species, Chlorovirus ATCV-1, commonly found in lakes, has been recently found to infect humans.[6] New studies focusing on effects of infection in mouse model are currently emerging as well.[6][7]
Taxonomy
Chlorovirus belongs to Group 1: dsDNA viruses, and is a genus of giant double stranded DNA, in the family of Phycodnaviridae.
Group: dsDNA
Family: Phycodnaviridae
Genus: Chlorovirus
- Acanthocystis turfacea chlorella virus 1
- Hydra viridis Chlorella virus 1
- Paramecium bursaria Chlorella virus 1, the type species
- Paramecium bursaria Chlorella virus A1
- Paramecium bursaria Chlorella virus AL1A
- Paramecium bursaria Chlorella virus AL2A
- Paramecium bursaria Chlorella virus BJ2C
- Paramecium bursaria Chlorella virus CA4A
- Paramecium bursaria Chlorella virus CA4B
- Paramecium bursaria Chlorella virus IL3A
- Paramecium bursaria Chlorella virus NC1A
- Paramecium bursaria Chlorella virus NE8A
- Paramecium bursaria Chlorella virus NY2A
- Paramecium bursaria Chlorella virus NYs1
- Paramecium bursaria Chlorella virus SC1A
- Paramecium bursaria Chlorella virus XY6E
- Paramecium bursaria Chlorella virus XZ3A
- Paramecium bursaria Chlorella virus XZ4A
- Paramecium bursaria Chlorella virus XZ4C
Ecology
Chloroviruses are widespread in freshwater environments in all parts of the globe and have been isolated from freshwater sources in Europe, Asia, Australia, as well as North and South America.[1][9] Natural hosts of chloroviruses include various types of unicellular eukaryotic Chlorella-like algae, with individual virus species typically infecting only within a distinct strain. These algal hosts are known to establish endosymbiotic relationships with larger protists, such as Paramecium bursaria (a member of the ciliates), Acanthocystis turfacea (a centroheliozoan) and Hydra viridis (member of the hydrozoa).[10] While an individual protist can harbour up to several hundred algal cells at any given time, free-floating algae are highly susceptible to chloroviruses, indicating that such endosymbiosis serves to provide resistance from infection.[11]
Chlorovirus titers are variable by season and location, but typically fluctuate between 1 and 100 PFU/mL, although high abundances of up to 100,000 PFU/mL may occur in some environments. Due to the rich genetic diversity and high specialization of individual species with respect to infectious range, variations in their ecology are not unusual, resulting in unique spatio-temporal patterns, which ultimately depend on lifestyle and nature of the host. As such, previous survey data highlighted two prominent seasonal abundance peaks for both Chlorella variabilis NC64A and Chlorella variabilis Syngen viruses — one in late fall, and the other in late spring to mid-summer — which is likely attributed to the fact that they share a host species. Conversely, Chlorella heliozoae SAG viruses peaked at different times of the year and generally exhibited more variability in titers, as compared to the NC64A and Syngen viruses.[1] Additionally, studies revealed that chloroviruses demonstrate some resilience in response to decreased temperatures observed during the winter season, characterized by presence of infectious particles under ice layers in a stormwater management pond in Ontario, Canada.[12] Further, DeLong et al. (2016) suggest that predation by small crustaceans can play an indirect role in titer fluctuations, as degradation of protist cells passing through the digestive tract results in liberation of large numbers of unicellular algae that become susceptible to viral infection due to disruption of endosymbiosis.[11] Overall, seasonal abundance of chloroviruses depends not only on the host species, but also on the abundance of other microorganisms, general nutrient status and ecological conditions.[13]
Collectively, chloroviruses are able to mediate global biogeochemical cycles through phytoplankton turnover. Chlorella, in co-occurrence with other types of microscopic algae like Microcystis aeruginosa, are known to cause toxic algal blooms that typically last from February to June in the Northern hemisphere, resulting in oxygen depletion and deaths of larger organisms in freshwater habitats.[14][15] Lytic infection of unicellular algae by chloroviruses results in termination of algal blooms and the subsequent release of carbon, nitrogen and phosphorus trapped in the cells, transporting them to lower trophic levels and, ultimately, fueling the food chain.[13]
Structure
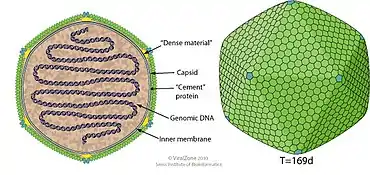
Viruses in the genus Chlorovirus are enveloped, with icosahedral and spherical geometries, and T=169 (triangulation number) symmetry. The diameter is around 100-220 nm. Genomes are linear, usually single-copy, composed of dsDNA (double-stranded DNA), and around 330 kb in length. The dsDNA is closed with a hairpin structure terminus. Genomes also often have several hundred open reading frames.[2] As a group, chloroviruses encode from 632 protein families; however, each individual virus only has 330 to 416 protein encoding genes. As part of the DNA modification systems, chloroviruses have methylated bases in specific sections of their DNA sequence. Some chloroviruses also contain introns and inteins, though this is rare within the genus.[10]
The type species PBCV-1 (Paramecium bursaria Chlorella virus 1) have a 190 nm diameter[10] and a fivefold axis.[16] One face's juncture has a protruding spike, which is the first part of the virus to contact its host.[17] The outer capsid covers a single lipid bilayer membrane, which is obtained from the host's endoplasmic reticulum.[16] Some capsomers on the external shell have fibres extending away from the virus to aid in host attachment.[18][17]
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Chlorovirus | Icosahedral | T=169 | Enveloped | Linear | Monopartite |
Hosts
Chloroviruses infect certain unicellular, eukaryotic chlorella-like green algae, called zoochlorellae, and are very species and even strain specific. These zoochlorellae commonly establish endosymbiotic relationships with the protozoan Paramecium bursaria, the coelenterate Hydra viridis, the heliozoon Acanthocystis turfacea and other freshwater and marine invertebrates and protozoans. The viruses cannot infect zoochlorellae when they are in their symbiotic phase, and there is no evidence that zoochlorellae grow free of their hosts in indigenous waters.[19] Chloroviruses have also recently been found to infect people, leading to studies on infections in mice as well.[6]
Life cycle
Viral replication is nucleo-cytoplasmic. Replication follows the DNA strand displacement model, and DNA-templated transcription is the method of transcription. The virus exits the host cell by lysis via lytic phospholipids, with passive diffusion being the mechanism behind transmission routes.
In three dimensional recreations of PBCV-1 (Paramecium bursaria chlorella virus), a prototype of chlorovirus, it is seen that the spike first contacts the host’s cell wall[20] and is aided by fibres in order to secure the virus to the host. The attachment of PBCV-1 to its receptor is very specific, and a major source of limitation with regards to viral host range. Virus-associated enzymes allow the host cell wall to degrade, and the viral internal membrane fuses with the host membrane. This fusion allows the transfer of viral DNA and virion-associated proteins into the host cell and also triggers depolarization of the host membrane. This is presumably occurring due to a virus encoded K+ channel. Studies predict this channel is within the virus, acting as an internal membrane releasing K+ from the cell, which may assist in the ejection of viral DNA and proteins from the viral cell to its host. The depolarization of the host’s cell membrane is also thought to prevent secondary infection from another virus or secondary transporters.
.png.webp)
Because PBCV-1 does not have an RNA polymerase gene, its DNA and viral-associated proteins move to the nucleus where transcription begins 5–10 minutes post infection. This rapid transcription is attributed to some component facilitating this transfer or viral DNA to the nucleus. This component is assumed to be a product of the PBCV-a443r gene, which obtains structures resembling proteins involved in nuclear trafficking in mammalian cells.
Host transcription rates decrease in this early phase of infection, and host transcription facilitators are reprogrammed to transcribe the new viral DNA. Minutes after infection, host chromosomal DNA degradation begins. This is presumed to occur through PBCV-1 encoded and packaged DNA restriction endonucleases. Degradation of the host chromosomal DNA inhibits host transcription. This results in 33-55% of the polyadenylated mRNAs in the infected cell being of viral origin by 20 minutes after initial infection.[22]
Viral DNA replication initiates after 60 to 90 minutes, which is then followed by the transcription of late genes within the host cell. Roughly 2–3 hours post infection, the assembly of virus capsids begins. This occurs within localized regions of the cytoplasm, with the virus capsids becoming prominent 3–4 hours after initial infection. 5–6 hours after PBCV-1 infection, the cytoplasm of the host cell fills with infectious progeny virus particles. Shortly after that (6–8 hours post infection), localized lysis of the host cell releases progeny. ~1000 particles are released from each infected cell, ~30% of which form plaques.[21]
Effects of infection
In algae infected with Cloroviruses the result is lysis, and thus death. As such, Chloroviruses are an important mechanism to the termination of algal blooms and play a vital role in the supply of nutrients to the water column[18] (See Ecology section for more information). Chloroviruses are also able to change the wall structure of infected cells. Some chloroviruses contain chitin synthase (CHS) genes while some others contain hyaluronan synthase (HAS) genes, respectively triggering the formation of chitin sensitive fibres or hyaluronan sensitive fibres. Though the function of producing a fibrous mat is not definitively known, it is believed that the fibres could: deter the uptake of the infected cell by symbiotic protozoans, which cause the digestion of the lysed cell; infect another host that takes up the fibre covered algae; or join with other infected and fibre covered cells. The ability to encode enzymes that trigger the synthesis of hyaluronan (hyaluronic acid) is found in no other viruses.[23]
Recently, chlorovirus ATCV-1 DNA has been found in human oropharyngeal samples. Prior to this is it was not known chlorovirus could infect humans, so there is limited knowledge about infections in people. People who were found to be infected had delayed memory and decreased attention. Humans found to be infected with ATCV-1 showed a decreased visual processing ability and reduced visual motor speed. This led to an overall decline in the ability to perform tasks based on vision and spatial reasoning.[6]
Studies infecting mice with ACTV-1 have been performed following the discovery chlorovirus can infect humans. The studies conducted on infected mice show changes in the Cdk5 pathway, which aids with learning and memory formation, as well as alterations in gene expression in the dopamine pathway.[6] Further, infected mice were found to be less social, interacting less with newly introduced companion mice than the control group. Infected mice also spent longer in a light-exposed portion of a test chamber, where the control mice tended to prefer the dark side and avoided the light. This indicates a decrease in anxiety with ACTV-1 infection. The test mice were also less able to recognize an object that had been moved from its previous location, showing a decrease in spatial reference memory.[7] As in humans, there is a decrease in vision spatial task ability. Within the hippocampus (area of brain responsible for memory and learning), changes in gene expression occur, and infection presents a change in the pathways of immune cell functioning and antigen processing. It has been suggested that this possibly indicates an immune system response to the ACTV-1 virus causing inflammation which may be the cause for the cognitive impairments.[6] The symptoms presented may also suggest hippocampus and medial prefrontal cortex interference from ACTV-1 infection.[7]
Evolution
Chloroviruses, as well as the remaining members of the family Phycodnaviridae, are considered part of the broader group of microbes called nucleocytoplasmic large DNA viruses (NCLDVs). Although phycodnaviruses are diverse genetically and infect different hosts, they display high levels of similarity on the structural level to each other and other NCLDVs. Phylogenetic analysis of the major capsid protein within the group indicates great likelihood of close relatedness, as well as prior divergence from a single common ancestor, which is believed to be a small DNA virus.[24][25] Additionally, studies suggest that genome gigantism, characteristic of all chloroviruses, is a property which evolved early on in the history of NCLDVs, and subsequent adaptations towards respective hosts and particular habitats resulted in mutations and gene loss events, which ultimately shaped all currently existing chlorovirus species.[25]
Genome sequencing and functional screening of proteins from PBCV-1 and ATCV-1 revealed large number of horizontally transferred genes, which indicates a long history of co-evolution with the unicellular host and lateral gene transfer with other seemingly unrelated organisms.[25] Further, both viruses were found to encode several so-called "progenitor enzymes", which are smaller, but less specialized than their modern-day analogues. For example, one of the sugar-manipulating enzymes in PBCV-1 (GDP-d-mannose 4,6 dehydratase or GMD) was shown to mediate catalysis of not only the dehydration of GDP-d-mannose, but also reduction of the sugar molecule produced in the initially predicted process. Such dual functionality is uncommon among the currently existing sugar-manipulating enzymes, and possibly suggests the ancient nature of the PBCV-1 GMD.[26]
Infection cycle studies in PBCV-1 revealed that the virus relies on a unique capsid glycosylation process independent of the host's ER or Golgi machinery. This feature has not yet been observed in any other virus currently known to science and potentially represents an ancient and conserved pathway, which could have evolved before eukaryogenesis, which was estimated to occur around 2.0-2.7 billion years ago.[26]
Recent discovery regarding presence of DNA sequences homologous to ATCV-1 in the human oropharyngeal virome, as well as the subsequent studies demonstrating successful infection of mammalian animal model by ATCV-1, also point to the likelihood of ancient evolutionary history of chloroviruses, which possess structural features and utilize molecular mechanisms that potentially allow for replication within diverse animal hosts.[6][27][28]
See also
- Phycodnaviridae - algae infecting viruses
References
- Quispe CF, Sonderman O, Seng A, Rasmussen B, Weber G, Mueller C, Dunigan DD, Van Etten JL (July 2016). "Three-year survey of abundance, prevalence and genetic diversity of chlorovirus populations in a small urban lake". Archives of Virology. 161 (7): 1839–47. doi:10.1007/s00705-016-2853-4. PMID 27068168.
- "Viral Zone". ExPASy. Retrieved 15 June 2015.
- ICTV. "Virus Taxonomy: 2014 Release". Retrieved 15 June 2015.
- Meints, Russel H.; Van Etten, James L.; Kuczmarski, Daniel; Lee, Kit; Ang, Barbara (September 1981). "Viral infection of the symbiotic chlorella-like alga present in Hydra viridis". Virology. 113 (2): 698–703. doi:10.1016/0042-6822(81)90198-7. PMID 18635088.
- Hoshina, Ryo; Shimizu, Mayumi; Makino, Yoichi; Haruyama, Yoshihiro; Ueda, Shin-ichiro; Kato, Yutaka; Kasahara, Masahiro; Ono, Bun-ichiro; Imamura, Nobutaka (13 September 2010). "Isolation and characterization of a virus (CvV-BW1) that infects symbiotic algae of Paramecium bursaria in Lake Biwa, Japan". Virology Journal. 7: 222. doi:10.1186/1743-422X-7-222. ISSN 1743-422X. PMC 2949830. PMID 20831832.
- Yolken RH, Jones-Brando L, Dunigan DD, Kannan G, Dickerson F, Severance E, Sabunciyan S, Talbot CC, Prandovszky E, Gurnon JR, Agarkova IV, Leister F, Gressitt KL, Chen O, Deuber B, Ma F, Pletnikov MV, Van Etten JL (November 2014). "Chlorovirus ATCV-1 is part of the human oropharyngeal virome and is associated with changes in cognitive functions in humans and mice". Proceedings of the National Academy of Sciences of the United States of America. 111 (45): 16106–11. doi:10.1073/pnas.1418895111. PMC 4234575. PMID 25349393.
- Petro, Marilyn S.; Agarkova, Irina V.; Petro, Thomas M. (August 2016). "Effect of Chlorovirus ATCV-1 infection on behavior of C57Bl/6 mice". Journal of Neuroimmunology. 297: 46–55. doi:10.1016/j.jneuroim.2016.05.009. PMID 27397075.
- ICTV. "Virus Taxonomy: 2014 Release". Retrieved 15 June 2015.
- Short SM (September 2012). "The ecology of viruses that infect eukaryotic algae". Environmental Microbiology. 14 (9): 2253–71. doi:10.1111/j.1462-2920.2012.02706.x. PMID 22360532.
- Van Etten JL, Dunigan DD (August 2016). "Giant Chloroviruses: Five Easy Questions". PLoS Pathogens. 12 (8): e1005751. doi:10.1371/journal.ppat.1005751. PMC 4990331. PMID 27536965.
- DeLong JP, Al-Ameeli Z, Duncan G, Van Etten JL, Dunigan DD (November 2016). "Predators catalyze an increase in chloroviruses by foraging on the symbiotic hosts of zoochlorellae". Proceedings of the National Academy of Sciences of the United States of America. 113 (48): 13780–13784. doi:10.1073/pnas.1613843113. PMC 5137705. PMID 27821770.
- Long AM, Short SM (July 2016). "Seasonal determinations of algal virus decay rates reveal overwintering in a temperate freshwater pond". The ISME Journal. 10 (7): 1602–12. doi:10.1038/ismej.2015.240. PMC 4918447. PMID 26943625.
- Yanai GM (2009). Transcription analysis of the chlorovirus Paramecium bursaria chlorella virus-1 (PhD). University of Nebraska at Lincoln.
- Song H, Lavoie M, Fan X, Tan H, Liu G, Xu P, Fu Z, Paerl HW, Qian H (August 2017). "Allelopathic interactions of linoleic acid and nitric oxide increase the competitive ability of Microcystis aeruginosa". The ISME Journal. 11 (8): 1865–1876. doi:10.1038/ismej.2017.45. PMC 5520033. PMID 28398349.
- Rieper M (1 March 1976). "Investigations on the relationships between algal blooms and bacterial populations in the Schlei Fjord (western Baltic Sea)". Helgoländer Wissenschaftliche Meeresuntersuchungen. 28 (1): 1–18. doi:10.1007/bf01610792. ISSN 0017-9957.
- Quispe, Cristian F.; Esmael, Ahmed; Sonderman, Olivia; McQuinn, Michelle; Agarkova, Irina; Battah, Mohammed; Duncan, Garry A.; Dunigan, David D.; Smith, Timothy P.L.; De Castro, Cristina; Speciale, Immacolata; Ma, Fangrui; Van Etten, James L. (January 2017). "Characterization of a new chlorovirus type with permissive and non-permissive features on phylogenetically related algal strains". Virology. 500: 103–113. doi:10.1016/j.virol.2016.10.013. PMC 5127778. PMID 27816636.
- Van Etten, James L.; Dunigan, David D. (January 2012). "Chloroviruses: not your everyday plant virus". Trends in Plant Science. 17 (1): 1–8. doi:10.1016/j.tplants.2011.10.005. PMC 3259250. PMID 22100667.
- Van Etten, James L.; Dunigan, David D.; Condit, Richard C. (18 August 2016). "Giant Chloroviruses: Five Easy Questions". PLOS Pathogens. 12 (8): e1005751. doi:10.1371/journal.ppat.1005751. PMC 4990331. PMID 27536965.
- Van Etten JL, Dunigan DD (January 2012). "Chloroviruses: not your everyday plant virus". Trends in Plant Science. 17 (1): 1–8. doi:10.1016/j.tplants.2011.10.005. PMC 3259250. PMID 22100667.
- Zhang, X; Xiang, Y; Dunigan, DD; Klose, T; Chipman, PR; Van Etten, JL; Rossmann, MG (2011). "Three dimensional structure and function of the Paramecium bursaria chlorella virus capsid". Proc. Natl. Acad. Sci. USA. 2011 (108): 14837–14842. doi:10.1073/pnas.1107847108. PMC 3169150. PMID 21873222.
- Van Etten, James L.; Dunigan, David D. (2012). "Chloroviruses: not your everyday plant virus". Trends in Plant Science. 17 (1): 1–8. doi:10.1016/j.tplants.2011.10.005. PMC 3259250. PMID 22100667.
- Blanc, G; Mozar, M; Agarkova, IV; Gurnon, JR; Yanai Balser, G; Rowe, JM; Xia, Y; Riethoven, JJ; Dunigan, DD; Van Etten, JL (2014). "Deep RNA sequencing reveals hidden features and dynamics of early gene transcription in Paramecium bursaria chlorella virus 1". PLoS ONE. 9: e90989. doi:10.1371/journal.pone.0090989. PMC 3946568. PMID 24608750.
- KANG, MING; DUNIGAN, DAVID D.; ETTEN, JAMES L. VAN (1 May 2005). "Chlorovirus: a genus of Phycodnaviridae that infects certain chlorella-like green algae". Molecular Plant Pathology. 6 (3): 213–224. doi:10.1111/j.1364-3703.2005.00281.x. PMID 20565652.
- Yutin N, Wolf YI, Koonin EV (October 2014). "Origin of giant viruses from smaller DNA viruses not from a fourth domain of cellular life". Virology. 466–467: 38–52. doi:10.1016/j.virol.2014.06.032. PMC 4325995. PMID 25042053.
- Dunigan DD, Fitzgerald LA, Van Etten JL (April 2006). "Phycodnaviruses: a peek at genetic diversity". Virus Research. 117 (1): 119–32. doi:10.1016/j.virusres.2006.01.024. PMID 16516998.
- Van Etten JL, Agarkova I, Dunigan DD, Tonetti M, De Castro C, Duncan GA (April 2017). "Chloroviruses Have a Sweet Tooth". Viruses. 9 (4): 88. doi:10.3390/v9040088. PMC 5408694. PMID 28441734.
- Petro TM, Agarkova IV, Zhou Y, Yolken RH, Van Etten JL, Dunigan DD (December 2015). "Response of Mammalian Macrophages to Challenge with the Chlorovirus Acanthocystis turfacea Chlorella Virus 1". Journal of Virology. 89 (23): 12096–107. doi:10.1128/JVI.01254-15. PMC 4645302. PMID 26401040.
- Petro MS, Agarkova IV, Petro TM (August 2016). "Effect of Chlorovirus ATCV-1 infection on behavior of C57Bl/6 mice". Journal of Neuroimmunology. 297: 46–55. doi:10.1016/j.jneuroim.2016.05.009. PMID 27397075.