Cilium
The cilium (from Latin 'eyelash';[1] the plural is cilia) is an organelle found on eukaryotic cells in the shape of a slender protuberance that projects from the much larger cell body.[2]
| Cilium | |
|---|---|
 SEM micrograph of the cilia projecting from respiratory epithelium in the lungs | |
| Details | |
| Identifiers | |
| Latin | Cilium |
| MeSH | D002923 |
| TH | H1.00.01.1.01014 |
| Anatomical terms of microanatomy | |
There are two types of cilia: motile and non-motile cilia. Non-motile cilia are also called primary cilia which serve as sensory organelles. Most mammalian cell types possess a single non-motile, primary cilium, which functions as a cellular antenna.[3][4] Exceptions include olfactory neurons which possess several non-motile cilia and cells of the transient embryonic node, which possess singular motile cilia known as nodal cilia, critical for the establishment of left to right body asymmetry.[5]
In eukaryotes, motile cilia and flagella (together known as undulipodia) are structurally similar, although distinctions are sometimes made according to function or length.[6][7] Immotile cilia (called primary cilia) communicate signals from the environment or from other cells.[8][9]
Types
Primary cilia
In animals, non-motile primary cilia are found on nearly every type of cell, blood cells being a prominent exception.[2] Most cells only possess one, in contrast to cells with motile cilia, an exception being olfactory sensory neurons, where the odorant receptors are located, which each possess about ten cilia. Some cell types, such as retinal photoreceptor cells, possess highly specialized primary cilia.[10]
Although the primary cilium was discovered in 1898, it was largely ignored for a century and considered a vestigial organelle without important function.[11][2] Recent findings regarding its physiological roles in chemosensation, signal transduction, and cell growth control, have revealed its importance in cell function. Its importance to human biology has been underscored by the discovery of its role in a diverse group of diseases caused by the dysgenesis or dysfunction of cilia, such as polycystic kidney disease,[12] congenital heart disease,[13] and retinal degeneration,[14] called ciliopathies.[15][16] The primary cilium is now known to play an important role in the function of many human organs.[2][3]
Cilia are assembled during the g1 phase and are disassembled before mitosis occurs.[17] Disassembly of cilia requires the action of the Aurora A kinase.[18] The current scientific understanding of primary cilia views them as "sensory cellular antennae that coordinate many cellular signaling pathways, sometimes coupling the signaling to ciliary motility or alternatively to cell division and differentiation."[19] The cilium is composed of subdomains and enclosed by a plasma membrane continuous with the plasma membrane of the cell. For many cilia, the basal body, where the cilium originates, is located within a membrane invagination called the ciliary pocket. The cilium membrane and the basal body microtubules are connected by distal appendages (also called transition fibers). Vesicles carrying molecules for the cilia dock at the distal appendages. Distal to the transition fibers form a transition zone where entry and exit of molecules is regulated to and from the cilia. Some of the signaling with these cilia occur through ligand binding such as Hedgehog signaling.[20] Other forms of signaling include G-coupled receptors including the somatostatin receptor 3 in neuronal cells.[21]
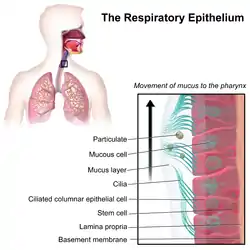
Motile cilia
Larger eukaryotes, such as mammals, have motile cilia as well. Motile cilia are usually present on a cell's surface in large numbers and beat in coordinated waves.[22]
- In humans, for example, motile cilia are found on the respiratory epithelium lining the respiratory tract where they function in the mucociliary clearance of sweeping mucus and dirt out of the lungs.[23] Each cell in the respiratory epithelium has around 200 motile cilia.[5]
- In female mammals, the beating of cilia in the Fallopian tubes moves the ovum from the ovary to the uterus.[23][24]
Motile cilia are also found on the epithelial cells of the choroid plexus epithelial cells. They are present in large numbers on each cell and move relatively slowly, making them intermediate between motile and primary cilia. In additional to 9+0 cilia that are mobile, there are also 9+2 cilia that stay immobile found in hair cells.[25]
The functioning of motile cilia is strongly dependent on the maintenance of optimal levels of periciliary fluid bathing the cilia. Epithelial sodium channels ENaC that are specifically expressed along the entire length of cilia apparently serve as sensors that regulate fluid level surrounding the cilia.[23][26]
Ciliates are microscopic organisms that possess motile cilia exclusively and use them for either locomotion or to simply move liquid over their surface.
Nodal cilia
The third type of cilium is a motile 9+0 cilium known as a nodal cilium. The nodal cilia are only present in the early development of the embryo. It is of similar structure to the primitive cilium in having no central apparatus, but it does possess dynein arms that enable it to move or spin in a circular direction.[5] The spin of a nodal cilium is clockwise, and this causes a flow of extraembryonic fluid to move across the nodal surface, directed to the left. Primary cilia around the nodal cilia sense the directional flow — which activates nodal signaling, establishing left to right sidedness.[5]
Structure

Inside cilia and flagella is a microtubule-based cytoskeleton called the axoneme. The axoneme of a primary cilium typically has a ring of nine outer microtubule doublets (called a 9+0 axoneme), and the axoneme of a motile cilium has, in addition to the nine outer doublets, two central microtubule singlets (called a 9+2 axoneme). The axoneme acts as a scaffold for axonemal inner and outer dynein arms that move motile cilia, and provides tracks for molecular motor proteins, such as Kinesin II, that carry proteins along the length of the cilium through a process called intraflagellar transport (IFT).[2][27][28] IFT is bi-directional and retrograde IFT employ the cytoskeletal dynein motor 2 to move back toward the cell body.The cilium is surrounded by a membrane contiguous with, but compositionally distinct from, the plasma membrane.[29]
The foundation of the cilium is the basal body, a term applied to the mother centriole when it is associated with a cilium. Mammalian basal bodies consist of a barrel of nine triplet microtubules, subdistal appendages and nine strut-like structures, known as distal appendages, which attach the basal body to the membrane at the base of the cilium. Two of the basal body's triplet microtubules extend to become the doublet microtubules of the ciliary axoneme.
Ciliary rootlet
The ciliary rootlet is a cytoskeleton-like structure that originates from the basal body at the proximal end of a cilium. Rootlets are typically 80-100 nm in diameter and contain cross striae distributed at regular intervals of approximately 55-70 nm. A prominent component of the rootlet is Rootletin.[30]
Transition zone
To achieve its distinct composition, the proximal-most region of the cilium consists of a transition zone that controls which proteins can enter and leave the cilium.[31][32][33] At the transition zone, Y-shaped structures connect the ciliary membrane to the underlying axoneme. Control of selective entry into cilia may involve a sieve-like function of transition zone. Inherited defects in components of the transition zone cause ciliopathies, such as Joubert syndrome. Transition zone structure and function is conserved across diverse organisms, including vertebrates, C. elegans, D. melanogaster and Chlamydomonas reinhardtii. In mammals, disruption of the transition zone reduces the ciliary abundance of membrane-associated ciliary proteins, such as those involved in Hedgehog signal transduction, compromising Hedgehog-dependent embryonic development of digit number and central nervous system patterning.
Cilia versus flagella
Though they have been given different names, motile cilia and flagella have nearly identical structures and have the same purpose: motion. The movement of the appendage can be described as a wave. The wave tends to originate from the cilium base and can be described in terms of frequency (ciliary beat frequency or CBF), amplitude and wave length. The beating motion is created by dynein arm structures the sliding of outer doublets, and originates in the axoneme, not at the basal body. A key difference between the two structures is that in a eukaryotic organism such as humans, flagella are used to propel the cell, while cilia are used to move substances across a surface. An example of each would be the flagellum present on a sperm cell and the cilium on the epithelial tissue of the lungs that clears out foreign particles. Motile cilia and flagella possess the same 9+2 axoneme structure. The 9 indicates the number of doublets present around the outer edge of the appendage while the 2 refers to a central pair of independent microtubules. In primary and other non-motile cilia, the axoneme lacks a central pair, resulting in a 9+0 axoneme structure.[34]
Cilium production
Cilia are formed through the process of ciliogenesis. An early step is docking of the basal body to the growing ciliary membrane, after which the transition zone forms. The building blocks of the ciliary axoneme, such as tubulins, are added at the ciliary tips through a process that depends partly on intraflagellar transport (IFT).[35][36] Exceptions include Drosophila sperm and Plasmodium falciparum flagella formation, in which cilia assemble in the cytoplasm.[37]
At the base of the cilium where it attaches to the cell body is the microtubule organizing center, the basal body. Some basal body proteins as CEP164, ODF2 [38] and CEP170,[39] are required for the formation and the stability of the cilium.
In effect, the cilium is a nanomachine composed of perhaps over 600 proteins in molecular complexes, many of which also function independently as nanomachines. Flexible linkers allow the mobile protein domains connected by them to recruit their binding partners and induce long-range allostery via protein domain dynamics.[19]
Function
The dynein in the axoneme forms bridges between neighbouring microtubule doublets. When ATP activates the motor domain of dynein, it attempts to walk along the adjoining microtubule doublet. This would force the adjacent doublets to slide over one another if not for the presence of Nexin between the microtubule doublets. And thus the force generated by dynein is instead converted into a bending motion.[40]
Sensing the extracellular environment
Some primary cilia on epithelial cells in eukaryotes act as cellular antennae, providing chemosensation, thermosensation and mechanosensation of the extracellular environment.[41][3] These cilia then play a role in mediating specific signalling cues, including soluble factors in the external cell environment, a secretory role in which a soluble protein is released to have an effect downstream of the fluid flow, and mediation of fluid flow if the cilia are motile.[41] Some epithelial cells are ciliated, and they commonly exist as a sheet of polarized cells forming a tube or tubule with cilia projecting into the lumen. This sensory and signalling role puts cilia in a central role for maintaining the local cellular environment and may be why ciliary defects cause such a wide range of human diseases.[16] In the mouse embryo, cilia are used to direct the flow of extracellular fluid. This leftward movement is used by the mouse embryo to generate left-right asymmetry across the midline of the embryo. Central cilia coordinate their rotational beating while the immotile cilia on the sides sense the direction of the flow.[42]
Clinical significance
Ciliary defects can lead to a number of human diseases.[16][43] Genetic mutations compromising the proper functioning of cilia, ciliopathies, can cause chronic disorders such as primary ciliary dyskinesia (PCD), nephronophthisis or Senior–Løken syndrome. In addition, a defect of the primary cilium in the renal tubule cells can lead to polycystic kidney disease (PKD). In another genetic disorder called Bardet–Biedl syndrome (BBS), the mutant gene products are the components in the basal body and cilia.[15]
Lack of functional cilia in the fallopian tubes can cause ectopic pregnancy. A fertilized ovum may not reach the uterus if the cilia are unable to move it there. In such a case, the ovum will implant in the fallopian tubes, causing a tubal pregnancy, the most common form of ectopic pregnancy.[44]
As noted above, epithelial sodium channels ENaC that are expressed along the length of cilia regulate fluid level surrounding the cilia. Mutations that decrease the activity of ENaC result in multisystem pseudohypoaldosteronism, that is associated with fertility problems.[23] In cystic fibrosis that results from mutations in the chloride channel CFTR, ENaC activity is enhanced leading to a severe reduction of the fluid level that causes complications and infections in the respiratory airways.[26]
Since the flagellum of human sperm is actually a modified cilium, ciliary dysfunction can also be responsible for male infertility.[45]
Of interest, there is an association of primary ciliary dyskinesia with left-right anatomic abnormalities such as situs inversus (a combination of findings known as Kartagener's syndrome) and other heterotaxic defects. These left-right anatomic abnormalities can also result in congenital heart disease.[46] It has been shown that proper cilial function is responsible for the normal left-right asymmetry in mammals.[47]
Ciliopathies as exemplars of multi-organ inherited diseases
Early 2000s findings in genetic research have suggested that many genetic disorders, both genetic syndromes and genetic diseases, that were not previously related in the medical literature, may be, in fact, highly related in the root cause of the widely varying set of medical symptoms that are clinically visible in the disorder. These have been grouped as an emerging class of diseases called ciliopathies. The underlying cause may be a dysfunctional molecular mechanism in the primary/immotile cilia, organelles which are present in many diverse cellular types throughout the human body.
Cilia defects adversely affect numerous critical signaling pathways essential to embryonic development and adult physiology, and thus offer a plausible hypothesis for the often multi-symptom nature of diverse ciliopathies.[15][16] Known ciliopathies include primary ciliary dyskinesia, Bardet–Biedl syndrome, polycystic kidney and liver disease, nephronophthisis, Alström syndrome, Meckel–Gruber syndrome, Sensenbrenner syndrome and some forms of retinal degeneration.[15][41]
The diverse outcomes caused by ciliary dysfunction may result from alleles of different strengths that compromise ciliary functions in different ways or to different extents. Many ciliopathies are inherited in a Mendelian manner, but specific genetic interactions between distinct functional ciliary complexes, such as transition zone and BBS complexes, can alter the phenotypic manifestations of recessive ciliopathies.[48][49]
Extracellular changes
Reduction of cilia function can also result from infection. Research into biofilms has been increasing and has shown how bacteria can alter cilia. A biofilm is a community of bacteria of either the same or multiple species of bacteria. The cluster of cells secretes different factors which form an extracellular matrix. Cilia in the respiratory system is known to move mucus and pathogens out of the airways. It has been found that patients with biofilm positive infections have impaired cilia function. The impairment may present as decreased motion or reduction in the number of cilia. Though these changes result from an external source, they still effect the pathogenicity of the bacteria, progression of infection, and how it is treated.[50]
References
- Mosby’s Medical, Nursing and Allied Health Dictionary, Fourth Edition, Mosby-Year Book Inc., 1994, p. 336
- Gardiner MB (September 2005). "The Importance of Being Cilia" (PDF). HHMI Bulletin. 18 (2). Retrieved 26 July 2008.
- Singla, Veena; Reiter, Jeremy F. (4 August 2006). "The primary cilium as the cell's antenna: signaling at a sensory organelle". Science. 313 (5787): 629–633. Bibcode:2006Sci...313..629S. doi:10.1126/science.1124534. ISSN 1095-9203. PMID 16888132. S2CID 29885142.
- Pazour, Gregory J.; Witman, George B. (February 2003). "The vertebrate primary cilium is a sensory organelle". Current Opinion in Cell Biology. 15 (1): 105–110. doi:10.1016/s0955-0674(02)00012-1. ISSN 0955-0674. PMID 12517711.
- Horani, A; Ferkol, T (May 2018). "Advances in the Genetics of Primary Ciliary Dyskinesia". Chest. 154 (3): 645–652. doi:10.1016/j.chest.2018.05.007. PMC 6130327. PMID 29800551.
- Haimo LT, Rosenbaum JL (December 1981). "Cilia, flagella, and microtubules". The Journal of Cell Biology. 91 (3 Pt 2): 125s–130s. doi:10.1083/jcb.91.3.125s. PMC 2112827. PMID 6459327.
- A Dictionary of Biology , 2004, accessed 6 April 2010.
- Elliott, Kelsey H.; Brugmann, Samantha A. (1 March 2019). "Sending mixed signals: Cilia-dependent signaling during development and disease". Developmental Biology. 447 (1): 28–41. doi:10.1016/j.ydbio.2018.03.007. ISSN 1095-564X. PMC 6136992. PMID 29548942.
- Karen Field Murray (2009). Fibrocystic Diseases of the Liver. Springer. pp. 47–. ISBN 978-1-60327-523-1. Retrieved 25 November 2010.
- Wolfrum, U., & Schmitt, A. (2000). Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motility and the Cytoskeleton, 46(2), 95–107.
- Satir, Peter (2017). "CILIA: before and after". Cilia. 6: 1. doi:10.1186/s13630-017-0046-8. ISSN 2046-2530. PMC 5343305. PMID 28293419.
- Wagner CA (2008). "News from the cyst: insights into polycystic kidney disease". Journal of Nephrology. 21 (1): 14–16. PMID 18264930.
- Brueckner M (June 2007). "Heterotaxia, congenital heart disease, and primary ciliary dyskinesia". Circulation. 115 (22): 2793–95. doi:10.1161/CIRCULATIONAHA.107.699256. PMID 17548739.
- Chen, Holly Y.; Kelley, Ryan A.; Li, Tiansen; Swaroop, Anand (31 July 2020). "Primary cilia biogenesis and associated retinal ciliopathies". Seminars in Cell & Developmental Biology. doi:10.1016/j.semcdb.2020.07.013. ISSN 1096-3634. PMID 32747192.
- Badano JL, Mitsuma N, Beales PL, Katsanis N (2006). "The ciliopathies: an emerging class of human genetic disorders". Annual Review of Genomics and Human Genetics. 7: 125–48. doi:10.1146/annurev.genom.7.080505.115610. PMID 16722803.
- Reiter, Jeremy F.; Leroux, Michel R. (September 2017). "Genes and molecular pathways underpinning ciliopathies". Nature Reviews. Molecular Cell Biology. 18 (9): 533–547. doi:10.1038/nrm.2017.60. ISSN 1471-0080. PMC 5851292. PMID 28698599.
- Pan J, Snell W (June 2007). "The primary cilium: keeper of the key to cell division". Cell. 129 (7): 1255–57. doi:10.1016/j.cell.2007.06.018. PMID 17604715. S2CID 17712155.
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (June 2007). "HEF1-dependent Aurora A activation induces disassembly of the primary cilium". Cell. 129 (7): 1351–63. doi:10.1016/j.cell.2007.04.035. PMC 2504417. PMID 17604723.
- Satir P, Christensen ST (June 2008). "Structure and function of mammalian cilia". Histochemistry and Cell Biology. 129 (6): 687–93. doi:10.1007/s00418-008-0416-9. PMC 2386530. PMID 18365235.
- Wong, Sunny Y.; Reiter, Jeremy F. (2008). "The primary cilium at the crossroads of mammalian hedgehog signaling". Current Topics in Developmental Biology. 85: 225–260. doi:10.1016/S0070-2153(08)00809-0. ISSN 0070-2153. PMC 2653622. PMID 19147008.
- Wheway G, Nazlamova L, Hancock JT (2018). "Signaling through the Primary Cilium". Frontiers in Cell and Developmental Biology. 6: 8. doi:10.3389/fcell.2018.00008. PMC 5809511. PMID 29473038.
- Benjamin Lewin (2007). Cells. Jones & Bartlett Learning. p. 359. ISBN 978-0-7637-3905-8.
- Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A (March 2012). "Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways". Histochemistry and Cell Biology. 137 (3): 339–53. doi:10.1007/s00418-011-0904-1. PMID 22207244. S2CID 15178940.
- "Cilia in nature" (PDF). hitech-projects.com. 2007. Archived from the original (PDF) on 24 October 2009. Retrieved 28 July 2008.
- Takeda, Sen; Narita, Keishi (February 2012). "Structure and function of vertebrate cilia, towards a new taxonomy". Differentiation. 83 (2): S4–S11. doi:10.1016/j.diff.2011.11.002.
- Hanukoglu I, Hanukoglu A (April 2016). "Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases". Gene. 579 (2): 95–132. doi:10.1016/j.gene.2015.12.061. PMC 4756657. PMID 26772908.
- Rosenbaum JL, Witman GB (November 2002). "Intraflagellar transport". Nature Reviews. Molecular Cell Biology. 3 (11): 813–25. doi:10.1038/nrm952. PMID 12415299. S2CID 12130216.
- Scholey JM (January 2008). "Intraflagellar transport motors in cilia: moving along the cell's antenna". The Journal of Cell Biology. 180 (1): 23–29. doi:10.1083/jcb.200709133. PMC 2213603. PMID 18180368.
- Rohatgi R, Snell WJ (August 2010). "The ciliary membrane". Current Opinion in Cell Biology. 22 (4): 541–46. doi:10.1016/j.ceb.2010.03.010. PMC 2910237. PMID 20399632.
- "Ciliary Rootlet". Gene Ontology. Retrieved 13 June 2012.
- Garcia, Galo; Raleigh, David R.; Reiter, Jeremy F. (23 April 2018). "How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In". Current Biology. 28 (8): R421–R434. doi:10.1016/j.cub.2018.03.010. ISSN 1879-0445. PMC 6434934. PMID 29689227.
- Garcia-Gonzalo, Francesc R.; Reiter, Jeremy F. (1 February 2017). "Open Sesame: How Transition Fibers and the Transition Zone Control Ciliary Composition". Cold Spring Harbor Perspectives in Biology. 9 (2): a028134. doi:10.1101/cshperspect.a028134. ISSN 1943-0264. PMC 5287074. PMID 27770015.
- Gonçalves, João; Pelletier, Laurence (April 2017). "The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate". Molecules and Cells. 40 (4): 243–253. doi:10.14348/molcells.2017.0054. ISSN 0219-1032. PMC 5424270. PMID 28401750.
- Foi A, Salvo FD, Doctorovich F, Huck-Iriart C, Ramallo-López JM, Dürr M, Ivanović-Burmazović I, Stirnat K, Garbe S, Klein A (August 2018). "Synthesis and structural characterisation of unprecedented primary N-nitrosamines coordinated to iridium(iv)". Dalton Transactions. 47 (33): 11445–54. doi:10.1042/BCJ20170453. PMID 30065990.
- Johnson KA, Rosenbaum JL (December 1992). "Polarity of flagellar assembly in Chlamydomonas". The Journal of Cell Biology. 119 (6): 1605–11. doi:10.1083/jcb.119.6.1605. PMC 2289744. PMID 1281816.
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM (June 2011). "Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments". Nature Cell Biology. 13 (7): 790–98. doi:10.1038/ncb2268. PMC 3129367. PMID 21642982.
- Of cilia and silliness (more on Behe) – The Panda's Thumb Archived 17 October 2007 at the Wayback Machine
- Ishikawa H, Kubo A, Tsukita S, Tsukita S (May 2005). "Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia". Nature Cell Biology. 7 (5): 517–24. doi:10.1038/ncb1251. PMID 15852003. S2CID 35443570.
- Lamla S (22 January 2009). Functional characterisation of the centrosomal protein Cep170 (Ph.D.). Ludwig-Maximilians-Universität München.
- Alberts, Bruce (2002). Molecular Biology of the Cell.
- Adams M, Smith UM, Logan CV, Johnson CA (May 2008). "Recent advances in the molecular pathology, cell biology and genetics of ciliopathies". Journal of Medical Genetics. 45 (5): 257–67. doi:10.1136/jmg.2007.054999. PMID 18178628.
- Wolpert, Lewis; Tickle, Cheryll; Martinez Arias, Alfonso (2015). Principles of Development (5th ed.). Oxford University Press. p. 227.
- Braun, Daniela A.; Hildebrandt, Friedhelm (1 March 2017). "Ciliopathies". Cold Spring Harbor Perspectives in Biology. 9 (3): a028191. doi:10.1101/cshperspect.a028191. ISSN 1943-0264. PMC 5334254. PMID 27793968.
- Horne AW, Critchley HO (March 2012). "Mechanisms of disease: the endocrinology of ectopic pregnancy". Expert Reviews in Molecular Medicine. 14: e7. doi:10.1017/erm.2011.2. PMID 22380790.
- Ichioka K, Kohei N, Okubo K, Nishiyama H, Terai A (July 2006). "Obstructive azoospermia associated with chronic sinopulmonary infection and situs inversus totalis". Urology. 68 (1): 204.e5–7. doi:10.1016/j.urology.2006.01.072. PMID 16850538.
- Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, Ahrens P, Lange L, Morillas HN, Noone PG, Zariwala MA, Knowles MR (June 2007). "Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia". Circulation. 115 (22): 2814–21. doi:10.1161/CIRCULATIONAHA.106.649038. PMID 17515466.
- McGrath J, Brueckner M (August 2003). "Cilia are at the heart of vertebrate left-right asymmetry". Current Opinion in Genetics & Development. 13 (4): 385–92. doi:10.1016/S0959-437X(03)00091-1. PMID 12888012.
- Leitch, Carmen C.; Zaghloul, Norann A.; Davis, Erica E.; Stoetzel, Corinne; Diaz-Font, Anna; Rix, Suzanne; Alfadhel, Majid; Al-Fadhel, Majid; Lewis, Richard Alan; Eyaid, Wafaa; Banin, Eyal (April 2008). "Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome". Nature Genetics. 40 (4): 443–448. doi:10.1038/ng.97. ISSN 1546-1718. PMID 18327255. S2CID 5282929.
- Yee, Laura E.; Garcia-Gonzalo, Francesc R.; Bowie, Rachel V.; Li, Chunmei; Kennedy, Julie K.; Ashrafi, Kaveh; Blacque, Oliver E.; Leroux, Michel R.; Reiter, Jeremy F. (November 2015). "Conserved Genetic Interactions between Ciliopathy Complexes Cooperatively Support Ciliogenesis and Ciliary Signaling". PLOS Genetics. 11 (11): e1005627. doi:10.1371/journal.pgen.1005627. ISSN 1553-7404. PMC 4635004. PMID 26540106.
- Fastenberg JH, Hsueh WD, Mustafa A, Akbar NA, Abuzeid WM (December 2016). "Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies". World Journal of Otorhinolaryngology – Head and Neck Surgery. 2 (4): 219–29. doi:10.1016/j.wjorl.2016.03.002. PMC 5698538. PMID 29204570.
