Emoxypine
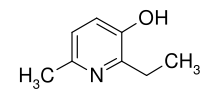
Emoxypine (2-ethyl-6-methyl-3-hydroxypyridine), also known as Mexidol or Mexifin when used as the succinate salt, is an antioxidant manufactured in Russia by Pharmasoft Pharmaceuticals.[2] Its chemical structure resembles that of pyridoxine (a type of vitamin B6). Being a Russia company, they did not seek approval for any medical use in the United States or Europe; similarly, US and EU companies do not seek approval for any medical use in Russia.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mexidol |
| Other names | Emoxipine, Emoxypin, Epigid, 6-Methyl-2-ethyl-3-hydroxypyridine |
| Routes of administration | Oral & IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 2-2.6 h |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.205.098 |
| Chemical and physical data | |
| Formula | C8H11NO |
| Molar mass | 137.182 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 170 to 172 °C (338 to 342 °F) [1] |
| |
| |
| (verify) | |
History
Emoxypine was first synthesized by L.D. Smirnov and K.M. Dumayev, then studied and developed in the Institute of Pharmacology, Russian Academy of Medical Sciences and National Scientific Center of Bioactive Substances Safety.[3]
Use
In Russia, emoxypine has a wide range of applications in medical practice. It purportedly exercises anxiolytic,[4][5] anti-stress, anti-alcohol, anticonvulsant, nootropic, neuroprotective and anti-inflammatory action. Emoxypine presumably improves cerebral blood circulation, inhibits thrombocyte aggregation, lowers cholesterol levels, has cardioprotective and antiatherosclerotic action.[3]
Mechanism of action
Emoxypine's mechanism of action is believed to be its antioxidant and membrane-protective effects with the following key components:[3][6]
- Emoxypine inhibits free radical oxidation of biomembrane lipids, reacts to peroxide radicals of lipids primary and hydroxyl radical of peptides
- Increases the activity of antioxidant enzymes, specifically that of superoxide dismutase, responsible for the formation and consumption of lipid peroxides and active oxygen forms
- Inhibits free radicals during the synthesis of prostaglandin catalyzed cyclooxygenase and lipoxygenase, increases the correlation prostacyclin/ thromboxane A2 and blocks the leukotriene formation
- Increases the content of polar fraction of lipids (phosphatidyl serine and phosphatidyl inositol) and reduces the cholesterol/phospholipids ratio which proves its lipid-regulatory properties; shifts structure transition into the low temperature zones, that is provokes the reduction of membrane viscosity and the increase of its fluidity, increases lipid-protein ratio.
- Modulates the activity of membrane-bound enzymes: phosphodiesterase, cyclic nucleotides, adenylate cyclase, aldoreductase, acetylcholinesterase.
- Modulates the receptor complexes of the brain membranes, i.e. benzodiazepine, GABA, acetylcholine receptors by increasing their binding ability.
- Stabilizes biomembranes, i.e. membrane structures of blood cells - erythrocytes and thrombocytes during their haemolysis or mechanical injury accompanied by the formation of free radicals.
- Changes the monoamine level and increases the dopamine content in the brain.[3][7]
Clinical study
One study determined the effectiveness of emoxypine in 205 patients with clinical manifestations of lumbosacral radiculopathy (LSR). Patients were divided into two groups, and further were divided into subgroups depending on the presence of motor disturbances. All patients received a course of conventional medical treatment and physiotherapy; main group additionally received emoxypine. Thereafter, clinical-neurological control of long-term results of treatment in subgroups of patients was performed. The results showed that the use of emoxypine in the combined therapy of patients with LSR led to significant and persistent reduction of severity of pain syndrome and rapid recovery of function of spinal roots and peripheral nerves compared with conventional therapy.[3][8]
References
- Gruber W (1953). "Synthesis of 3-Hydroxy-2-alkylpyridines". Canadian Journal of Chemistry. 31 (6): 564–568. doi:10.1139/v53-079.
- "mexidol.ru, Pharmasoft Website". Archived from the original on 2011-04-10. Retrieved 2011-04-27.
- Voronina TA. "ANTIOXIDANT MEXIDOL. The main neuropsychotropic effects and the mechanism of action". Institute of Pharmacology. Moscow, Russia: Russian Academy of Medical Sciences. Archived from the original on 2013-11-05.
- Volchegorskii IA, Miroshnichenko IY, Rassokhina LM, Faizullin RM, Malkin MP, Pryakhina KE, Kalugina AV (April 2015). "Comparative analysis of the anxiolytic effects of 3-hydroxypyridine and succinic acid derivatives". Bulletin of Experimental Biology and Medicine. 158 (6): 756–61. doi:10.1007/s10517-015-2855-3. PMID 25894772. S2CID 6052275.
- Rumyantseva SA, Fedin AI, Sokhova ON (October 2012). "Antioxidant Treatment of Ischemic Brain Lesions". Neuroscience and Behavioral Physiology. 42 (8): 842–845. doi:10.1007/s11055-012-9646-3. S2CID 39971165.
- Dumayev KM, Voronina TA, Smirnov LD (1995). Antioxidants in the prophylaxis and therapy of CNS pathologies. Moscow.
- Kucherianu VG (January 2001). "[Mexidol potentiates antiparkinsonian effect of L-DOPA in MPTP-induced parkinsonism model]". Eksperimental'naia i Klinicheskaia Farmakologiia. 64 (1): 22–5. PMID 11544797.
- Likhacheva EB, Sholomov II (2006). "[Clinical and immunological assessment of efficacy of mexidol in the treatment of lumbosacral radiculopathy]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova. 106 (10): 52–7. PMID 17117675.