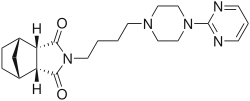
Tandospirone
Tandospirone (brand name Sediel) is an anxiolytic and antidepressant drug used in China and Japan, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone class of drugs and is closely related to other azapirones like buspirone and gepirone.
 | |
| Clinical data | |
|---|---|
| Trade names | Sediel |
| Other names | Metanopirone |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 2–3 hours (3–5 hours for metabolite 1-PP) |
| Excretion | Urine (70%; 0.1% as unchanged drug) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.461 |
| Chemical and physical data | |
| Formula | C21H29N5O2 |
| Molar mass | 383.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Medical uses
Anxiety and depression
Tandospirone is most commonly used as a treatment for anxiety and depressive disorders, such as generalised anxiety disorder and dysthymia respectively.[1] For both indications it usually takes a couple of weeks for therapeutic effects to begin to be seen,[1] although at higher doses more rapid anxiolytic responses have been seen.[2] It has also been used successfully as a treatment for bruxism.[3]
Other uses
Tandospirone has also been tried, successfully, as an adjunctive treatment for cognitive symptoms in schizophrenic individuals.[4]
Side effects
Common adverse effects include:[1]
- Dizziness
- Drowsiness
- Insomnia
- Headache
- Gastrointestinal disorders
- Dry mouth
- Negative influence on explicit memory function [1]
Adverse effects with unknown frequency include:[1]
- Hypotension (low blood pressure)
- Dysphoria
- Tachycardia
- Malaise
- Psychomotor impairment
It is not believed to be addictive but it is known to produce mild withdrawal effects (e.g. anorexia) after abrupt discontinuation.[1]
Pharmacology
Tandospirone acts as a potent and selective 5-HT1A receptor partial agonist, with a Ki affinity value of 27 ± 5 nM[5] and approximately 55 to 85% intrinsic activity.[6][7] It has weak and clinically negligible affinity for the 5-HT2A (1,300 ± 200), 5-HT2C (2,600 ± 60), α1-adrenergic (1,600 ± 80), α2-adrenergic (1,900 ± 400), D1 (41,000 ± 10,000), and D2 (1,700 ± 300) receptors, and is essentially inactive at the 5-HT1B, 5-HT1D, β-adrenergic, and muscarinic acetylcholine receptors, serotonin transporter, and benzodiazepine allosteric site of the GABAA receptor (all of which are > 100,000).[5] There is evidence of tandospirone having low but significant antagonistic activity at the α2-adrenergic receptor through its active metabolite 1-(2-pyrimidinyl)piperazine (1-PP).[8][9]
Research
In February 2018, a study was conducted by researchers at the Queensland University of Technology and published in the journal Scientific Reports. Researchers found that tandospirone helped reverse brain deficits caused by chronic alcohol exposure in mice.[10] The study was the first of its kind and brought interest in the drug for possible treatment of alcohol withdrawal symptoms.[11]
Society and culture
Name
Tandospirone has also been known as metanopirone.
References
- Barradell LB, Fitton A (February 1996). "Tandospirone". CNS Drugs. 5 (2): 147–153. doi:10.2165/00023210-199605020-00006.
- Nishitsuji K, To H, Murakami Y, Kodama K, Kobayashi D, Yamada T, et al. (2004). "Tandospirone in the treatment of generalised anxiety disorder and mixed anxiety-depression : results of a comparatively high dosage trial". Clinical Drug Investigation. 24 (2): 121–6. doi:10.2165/00044011-200424020-00007. PMID 17516698.
- Tandospirone. Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 23 September 2011. Retrieved 14 November 2013.
- Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, et al. (October 2001). "Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment". The American Journal of Psychiatry. 158 (10): 1722–5. doi:10.1176/appi.ajp.158.10.1722. PMID 11579010.
- Hamik A, Oksenberg D, Fischette C, Peroutka SJ (July 1990). "Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites". Biological Psychiatry. 28 (2): 99–109. doi:10.1016/0006-3223(90)90627-E. PMID 1974152.
- Tanaka H, Tatsuno T, Shimizu H, Hirose A, Kumasaka Y, Nakamura M (December 1995). "Effects of tandospirone on second messenger systems and neurotransmitter release in the rat brain". General Pharmacology. 26 (8): 1765–72. doi:10.1016/0306-3623(95)00077-1. PMID 8745167.
- Yabuuchi K, Tagashira R, Ohno Y (2004). "Effects of tandospirone, a novel anxiolytic agent, on human 5-HT1A receptors expressed in Chinese hamster ovary cells (CHO cells)". Biogenic Amines. 18 (3): 319. doi:10.1163/1569391041501933.
- Blier P, Curet O, Chaput Y, de Montigny C (July 1991). "Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine--II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission". Neuropharmacology. 30 (7): 691–701. doi:10.1016/0028-3908(91)90176-C. PMID 1681447.
- Miller LG, Thompson ML, Byrnes JJ, Greenblatt DJ, Shemer A (October 1992). "Kinetics, brain uptake, and receptor binding of tandospirone and its metabolite 1-(2-pyrimidinyl)-piperazine". Journal of Clinical Psychopharmacology. 12 (5): 341–5. doi:10.1097/00004714-199210000-00009. PMID 1362206.
- Belmer A, Patkar OL, Lanoue V, Bartlett SE (February 2018). "5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol". Scientific Reports. 8 (1): 2099. Bibcode:2018NatSR...8.2099B. doi:10.1038/s41598-018-20504-z. PMC 5794771. PMID 29391482.
- Burke G (2018-02-08). "New drug could reverses binge drinking effects on brain, help alcoholics: scientists". ABC.net.au. Retrieved 8 February 2018.