Mepiprazole
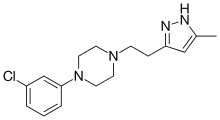
Mepiprazole (INN, BAN) (brand name Psigodal) is an anxiolytic drug of the phenylpiperazine group with additional antidepressant properties[1] that is marketed in Spain.[2][3][4][5][6] It acts as a 5-HT2A and α1-adrenergic receptor antagonist[7][8][9] and inhibits the reuptake and induces the release of serotonin, dopamine, and norepinephrine to varying extents,[1][9] and has been described as a serotonin antagonist and reuptake inhibitor (SARI).[10] Controlled clinical trials of mepiprazole in patients with irritable bowel syndrome (IBS) were also carried out and suggested some benefits of the drug in relieving symptoms of IBS in some patients.[11] Similarly to other phenylpiperazines like trazodone, nefazodone, and etoperidone, mepiprazole produces mCPP as an active metabolite.[12]
 | |
| Clinical data | |
|---|---|
| Trade names | Psigodal |
| Other names | PAP, EMD-16923, H-4007 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H21ClN4 |
| Molar mass | 304.82 g·mol−1 |
| 3D model (JSmol) | |
| |
See also
References
- Placheta P, Singer E, Kriwanek W, Hertting G (August 1976). "Mepiprazole, a new psychotropic drug: effects on uptake and retention of monoamines in rat brain synaptosomes". Psychopharmacology. 48 (3): 295–301. doi:10.1007/BF00496865. PMID 9660. S2CID 9194743.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 768–. ISBN 978-1-4757-2085-3.
- Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- Pöldinger W (1975). "Clinical trial of 3-methyl-5-(beta-N'-(N-m-chlorophenylpiperazino)ethyl)-pyrazole dihydrochloride (Mepiprazol) in the therapy of psychovegetative disorders". International Pharmacopsychiatry. 10 (1): 1–8. doi:10.1159/000468162. PMID 1095510.
- De Buck R, Van Durme R, Pelc I (May 1975). "[A controlled double-blind crossover study of the efficacy of mepiprazol (EMD 16.923) and of diazepam in the treatment of neurotic disorders]". Acta Psychiatrica Belgica (in French). 75 (3): 320–33. PMID 769484.
- Saldaña Hernández OH, Hernández González J (1976). "[Psychopharmological research with EMD 16-923 in patients with different degrees of anxiety]". Neurología, Neurocirugía, Psiquiatría (in Spanish). 17 (1): 29–33. PMID 1052713.
- Cohen ML, Fuller RW, Kurz KD (1983). "Evidence that blood pressure reduction by serotonin antagonists is related to alpha receptor blockade in spontaneously hypertensive rats". Hypertension. 5 (5): 676–81. doi:10.1161/01.hyp.5.5.676. PMID 6311738.
- Maj J, Sypniewska M (1980). "Central action of mepiprazole". Polish Journal of Pharmacology and Pharmacy. 32 (4): 475–84. PMID 7255266.
- Fuxe K, Agnati LF, Ungerstedt U (January 1976). "The effect of mepiprazole on central monoamine neurons. Evidence for increased 5-hydroxytryptamine and dopamine receptor activity". European Journal of Pharmacology. 35 (1): 93–108. doi:10.1016/0014-2999(76)90304-6. PMID 943291.
- Fagiolini, Andrea; Comandini, Alessandro; Dell’Osso, Mario Catena; Kasper, Siegfried (2012). "Rediscovering Trazodone for the Treatment of Major Depressive Disorder". CNS Drugs. 26 (12): 1033–1049. doi:10.1007/s40263-012-0010-5. ISSN 1172-7047. PMC 3693429. PMID 23192413.
- Dotevall G, Groll E (October 1974). "Controlled clinical trial of mepiprazole in irritable bowel syndrome". The British Medical Journal. 4 (5935): 16–8. doi:10.1136/bmj.4.5935.16. PMC 1612118. PMID 4609545.
- Fong MH, Garattini S, Caccia S (October 1982). "1-m-Chlorophenylpiperazine is an active metabolite common to the psychotropic drugs trazodone, etoperidone and mepiprazole". The Journal of Pharmacy and Pharmacology. 34 (10): 674–5. doi:10.1111/j.2042-7158.1982.tb04701.x. PMID 6128394. S2CID 44968564.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Simple piperazines (no additional rings) | |
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |