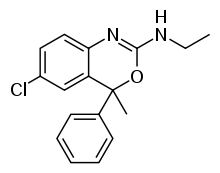
Etifoxine
Etifoxine (INN; also known as etafenoxine; trade name Stresam) is an anxiolytic and anticonvulsant drug[2][3] developed by Hoechst in the 1960s.[4] It is sold in approximately 40 countries for anxiety disorders, without the sedation and ataxia associated with benzodiazepine drugs.[5] It has similar anxiolytic effects to benzodiazepine drugs, but is structurally distinct, although it has structural elements in common with them.[6] Studies suggest is as effective as lorazepam as an anxiolytic, but has fewer side effects.[7] Etifoxine is not approved by the U.S. Food and Drug Administration or the European Medicines Agency.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Stresam |
| Other names | Etafenoxine |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (no CYP450 interactions) |
| Elimination half-life | 6 hours (etifoxine),[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.158.584 |
| Chemical and physical data | |
| Formula | C17H17ClN2O |
| Molar mass | 300.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Side effects
The most common adverse effect of etifoxine is mild drowsiness at initial dosing. It is not associated with any withdrawal syndromes or dependence. Etifoxine shows less adverse effects of anterograde amnesia, sedation, impaired psychomotor performance, and withdrawal syndromes than those of benzodiazepines.[1] A 2012 review of etifoxine by the French National Pharmacovigilance Committee determined that etifoxine was safe and continued to provide a favorable alternative to benzodiazepine anxiolytics. The committee found (for a ten-year pharmacovigilance period) that safety concerns were rare or very rare and that the incidence of idiosyncratic hepatic problems were very rare.[8]
Mechanism of action
Unlike benzodiazepines, etifoxine may produce its anxiolytic effects through a dual mechanism, by directly binding to GABAA receptors and (purportedly, exact binding site undetermined) to the mitochondrial translocator protein (TSPO), resulting in increases in endogenous neurosteroids.
At GABAA receptors etifoxine binds at the α+β− interface and preferentially potentiates α2β3γ2 and α3β3γ2 receptor types.[9] This direct allosteric potentiation can only be observed at relatively high concentrations (starting at > 1 micromolar) and is perhaps not physiologically relevant at normal human doses.[10] This is different than benzodiazepines and etifoxine can be used alongside benzodiazepines to potentiate their effects without competing for binding sites;[11] however, it also means that the direct effects of etifoxine are not reversed by the benzodiazepine antagonist flumazenil.[12]
Etifoxine stimulates the biosynthesis of endogenous neurosteroids, for example: allopregnanolone (3-alpha,5-alpha THP), a nanomolar potentiator of GABA activity.[13]
References
- Choi, Yun Mi; Kim, Kyung Hoon (2015). "Etifoxine for Pain Patients with Anxiety". The Korean Journal of Pain. 28 (1): 4–10. doi:10.3344/kjp.2015.28.1.4. PMC 4293506. PMID 25589941.
- Kruse, HJ; Kuch, H (1985). "Etifoxine: evaluation of its anticonvulsant profile in mice in comparison with sodium valproate, phenytoin and clobazam". Arzneimittel-Forschung. 35 (1): 133–5. PMID 2859023.
- The Merck Index, 12th Edition. 3910.
- U.S. Patent 3,725,404
- Girard C, Liu S, Cadepond F, Adams D, Lacroix C, Verleye M, Gillardin JM, Baulieu EE, Schumacher M, Schweizer-Groyer G (2008). "Etifoxine improves peripheral nerve regeneration and functional recovery". Proc Natl Acad Sci U S A. 105 (51): 20505–10. Bibcode:2008PNAS..10520505G. doi:10.1073/pnas.0811201106. PMC 2629330. PMID 19075249.
- Schlichter R, Rybalchenko V, Poisbeau P, Verleye M, Gillardin J (2000). "Modulation of GABAergic synaptic transmission by the non-benzodiazepine anxiolytic etifoxine". Neuropharmacology. 39 (9): 1523–35. doi:10.1016/s0028-3908(99)00253-1. PMID 10854897. S2CID 860299.
- Nguyen N, Fakra E, Pradel V, Jouve E, Alquier C, Le Guern ME, Micallef J, Blin O (2006). "Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: a double-blind controlled study in general practice". Human Psychopharmacology. 21 (3): 139–49. doi:10.1002/hup.757. PMID 16625522. S2CID 25940120.
- "COMMISSION NATIONALE DE PHARMACOVIGILANCE Compte rendu de la réunion du mardi 26 juin 2012" (PDF) (in French).
- Mattei C, Taly A, Soualah Z, Saulais O, Henrion D, Guérineau NC, Verleye M, Legros C (2019). "Involvement of the GABAA receptor α subunit in the mode of action of etifoxine" (PDF). Pharmacol. Res. 145: 104250. doi:10.1016/j.phrs.2019.04.034. PMID 31059790.
- Hamon A, Morel A, Hue B, Verleye M, Gillardin JM (2003). "The modulatory effects of etifoxine's direct effects on GABA(A) receptors are mediated by the beta subunit". Neuropharmacology. 45 (3): 293–303. doi:10.1016/s0028-3908(03)00187-4. PMID 12871647. S2CID 9892214.
- Kruse HJ, Kuch H (1986). "Potentiation of clobazam's anticonvulsant activity by etifoxine, a non-benzodiazepine tranquilizer, in mice. Comparison studies with sodium valproate". Arzneimittelforschung. 36 (9): 1320–2. PMID 3098254.
- Verleye M, Schlichter R, Gillardin JM (1999). "Interactions of etifoxine with the chloride channel coupled to the GABA(A) receptor complex". NeuroReport. 10 (15): 3207–10. doi:10.1097/00001756-199910190-00015. PMID 10574561.
- do Rego, Jean Luc; Vaudry, David; Vaudry, Hubert (2015). "The Non-Benzodiazepine Anxiolytic Drug Etifoxine Causes a Rapid, Receptor-Independent Stimulation of Neurosteroid Biosynthesis". PLOS ONE. 10 (3): e0120473. Bibcode:2015PLoSO..1020473D. doi:10.1371/journal.pone.0120473. PMC 4364751. PMID 25785994.
