Picamilon
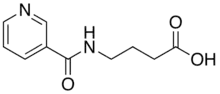
Picamilon (also known as N-nicotinoyl-GABA, pycamilon, and pikamilon) is a drug formed by a synthetic combination of niacin and γ-aminobutyric acid (GABA). It was developed in the Soviet Union in 1969[1] and further studied in both Russia[2] and Japan as a prodrug of GABA.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | НПК ЭХО |
| Other names | nicotinoyl-GABA |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50%–88% |
| Elimination half-life | 30 minutes |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.118.799 |
| Chemical and physical data | |
| Formula | C10H12N2O3 |
| Molar mass | 208.217 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
In Russia, picamilon is sold as a prescription drug. The rights to the drug belong to the Russian pharmaceutical company NPK ECHO ("НПК ЭХО"). It is not approved for sale in the United States and has been deemed an adulterating agent in dietary supplements,[4] with five American companies required to remove their picamilon products from the market in November, 2015.[5] However, as recently as 2020, picamilon has been found in pharmaceutical dosages in over-the-counter supplements in the US.[6]
Mechanism of action and potential therapeutic applications
One study in animals showed that picamilon permeated the blood–brain barrier[7] and then is hydrolyzed into GABA and niacin.[8] The released GABA in theory would activate GABA receptors potentially producing an anxiolytic response.[9] The second released component, niacin, is a vasodilator.[10][11]
Detection in biological fluids
Plasma picamilon concentrations are generally in the 500–3000 μg/L range during the first few hours after single oral doses of 50–200 mg with a half-life of 1–2 hours.[12] The drug undergoes hydrolysis to GABA and nicotinic acid. Urinary excretion of parent drug and the two metabolites accounts for up to 79% of a single dose.[12]

Regulation
In the United States, the Food and Drug Administration ruled in 2015 that picamilon does not fit any of the dietary ingredient categories in the Dietary Supplement Health and Education Act of 1994,[5][13] namely that it is not a vitamin; a dietary mineral; an herb or other botanical; an amino acid; a dietary substance for use by humans to supplement the diet by increasing the total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of any ingredient described above that had been marketed in the United States before 1994. Despite the FDA ruling, picamilon remains an ingredient in supplements marketed as nootropics in the US.[6]
References
- Kopelevich VM, Gunar VI (April 1999). "Some approaches to the directed search for new drugs based on nicotinic acid". Pharmaceutical Chemistry Journal. 33 (4): 177–187. doi:10.1007/BF02509934. S2CID 36930437.
- Mirzoian RS, Gan'shina TS (1989). "[The new cerebrovascular preparation pikamilon]". Farmakologiia I Toksikologiia (in Russian). 52 (1): 23–6. PMID 2707413.
- Matsuyama K, Yamashita C, Noda A, Goto S, Noda H, Ichimaru Y, Gomita Y (October 1984). "Evaluation of isonicotinoyl-gamma-aminobutyric acid (GABA) and nicotinoyl-GABA as pro-drugs of GABA". Chemical & Pharmaceutical Bulletin. 32 (10): 4089–95. doi:10.1248/cpb.32.4089. PMID 6529802.
- Avula B, Chittiboyina AG, Sagi S, Wang YH, Wang M, Khan IA, Cohen PA (March 2016). "Identification and quantification of vinpocetine and picamilon in dietary supplements sold in the United States". Drug Testing and Analysis. 8 (3–4): 334–43. doi:10.1002/dta.1853. PMID 26426301.
- "FDA sends five warning letters over supplements containing picamilon". NutraIngredients-USA.com, William Reed Business Media. 2 December 2015. Retrieved 3 December 2015.
- Cohen, Pieter A.; Avula, Bharathi; Wang, Yan Hong; Zakharevich, Igor; Khan, Ikhlas (23 September 2020). "Five unapproved drugs found in cognitive enhancement supplements". Neurology: Clinical Practice: 10.1212/CPJ.0000000000000960. doi:10.1212/CPJ.0000000000000960.
- Dorofeev BF, Kholodov LE (1991). "[Pikamilon pharmacokinetics in animals]". Farmakologiia I Toksikologiia (in Russian). 54 (2): 66–9. PMID 1884802.
- "Technical Description of Picamilon". Archived from the original on 2018-11-09. Retrieved 2015-10-22.
- Shephard RA (June 1987). "Behavioral effects of GABA agonists in relation to anxiety and benzodiazepine action". Life Sciences. 40 (25): 2429–36. doi:10.1016/0024-3205(87)90758-2. PMID 2884549.
- Gille A, Bodor ET, Ahmed K, Offermanns S (2008). "Nicotinic acid: pharmacological effects and mechanisms of action". Annual Review of Pharmacology and Toxicology. 48: 79–106. doi:10.1146/annurev.pharmtox.48.113006.094746. PMID 17705685.
- Prousky J, Seely D (January 2005). "The treatment of migraines and tension-type headaches with intravenous and oral niacin (nicotinic acid): systematic review of the literature". Nutrition Journal. 4: 3. doi:10.1186/1475-2891-4-3. PMC 548511. PMID 15673472.
- Cui W, Chen X, Zhan Y, Zhang Z, Zhang Y, Zhong D (May 2010). "Determination of picamilon concentration in human plasma by liquid chromatography-tandem mass spectrometry". Journal of Chromatography B. 878 (15–16): 1181–4. doi:10.1016/j.jchromb.2010.03.013. PMID 20359966.
- Welch C. "Declaration of Dr. Cara Welch" (PDF). Department of Health and Human Services. Retrieved 21 October 2015.
External links
- nicotinoyl-GABA at the US National Library of Medicine Medical Subject Headings (MeSH)
