Gemeprost
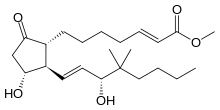
Gemeprost (16, 16-dimethyl-trans-delta2 PGE1 methyl ester) is an analogue of prostaglandin E1.
 | |
| Clinical data | |
|---|---|
| Trade names | Cervagem |
| Other names | methyl (E)-7-[(1R,2S,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4,4-dimethyl-oct-1-enyl]-5-oxo-cyclopentyl]hept-2-enoate |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Pessary |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.058.869 |
| Chemical and physical data | |
| Formula | C23H38O5 |
| Molar mass | 394.552 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Clinical use
It is used as a treatment for obstetric bleeding.
It is used with mifepristone to terminate pregnancy up to 24 weeks gestation.[2]
Side effects
Vaginal bleeding, cramps, nausea, vomiting, loose stools or diarrhea, headache, muscle weakness; dizziness; flushing; chills; backache; dyspnoea; chest pain; palpitations and mild pyrexia. Rare: Uterine rupture, severe hypotension, coronary spasms with subsequent myocardial infarctions.
References
- "Cervagem 1 mg Pessary - Summary of Product Characteristics (SmPC)". (emc). 16 August 2019. Retrieved 7 September 2020.
- Bartley J, Brown A, Elton R, Baird DT (October 2001). "Double-blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation". Human Reproduction (Oxford, England). 16 (10): 2098–102. doi:10.1093/humrep/16.10.2098. PMID 11574498. Retrieved 2008-10-29.
External links
- "Gemeprost". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.