Menatetrenone
Menatetrenone (INN), also known as MK-4, is one of the nine forms of vitamin K2.
 | |
 | |
| Clinical data | |
|---|---|
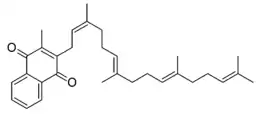
| Other names | 3-methyl-2-[(2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl]naphthalene-1,4-dione |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C31H40O2 |
| Molar mass | 444.659 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
MK-4 is produced via conversion of vitamin K1 in the body, in the testes, pancreas and arterial walls.[1] While major questions still surround the biochemical pathway for the transformation of vitamin K1 to MK-4, studies demonstrate the conversion is not dependent on gut bacteria, occurring in germ-free rats[2][3] and in parenterally-administered K1 in rats.[4][5] In fact, tissues that accumulate high amounts of MK-4 have a remarkable capacity to convert up to 90% of the available K1 into MK-4.[2][3]
Dose
Bioavailability studies have shown that small oral doses are not detected in the blood – for example 420 mcg of menatetrenone or less tested over minutes and hours are not detectable,[6] and larger doses in the order of milligrams are required, unlike vitamin K2 MK-7 which is detectable in the blood in mcg doses. Furthermore, "administered daily doses of 15, 45, 90, and 135 mg revealed that 45 mg was the minimum effective dose for improving bone mass parameters evaluated by microdensitometry and/or single photon absorptiometry in postmenopausal women with osteoporosis".[7]
See also
- Vitamin K
- Phylloquinone (vitamin K1)
References
- Shearer MJ, Newman P (October 2008). "Metabolism and cell biology of vitamin K". Thrombosis and Haemostasis. 100 (4): 530–47. doi:10.1160/TH08-03-0147. PMID 18841274.
- Davidson RT, Foley AL, Engelke JA, Suttie JW (February 1998). "Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria". The Journal of Nutrition. 128 (2): 220–3. doi:10.1093/jn/128.2.220. PMID 9446847.
- Ronden JE, Drittij-Reijnders MJ, Vermeer C, Thijssen HH (January 1998). "Intestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the rat". Biochimica et Biophysica Acta (BBA) - General Subjects. 1379 (1): 69–75. doi:10.1016/S0304-4165(97)00089-5. PMID 9468334.
- Thijssen HH, Drittij-Reijnders MJ (September 1994). "Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4". The British Journal of Nutrition. 72 (3): 415–25. doi:10.1079/BJN19940043. PMID 7947656.
- Will BH, Usui Y, Suttie JW (December 1992). "Comparative metabolism and requirement of vitamin K in chicks and rats". The Journal of Nutrition. 122 (12): 2354–60. doi:10.1093/jn/122.12.2354. PMID 1453219.
- Sato T, Schurgers LJ, Uenishi K (November 2012). "Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women". Nutrition Journal. 11 (93): 93. doi:10.1186/1475-2891-11-93. PMC 3502319. PMID 23140417.
- Iwamoto J (May 2014). "Vitamin K₂ therapy for postmenopausal osteoporosis". Nutrients. 6 (5): 1971–80. doi:10.3390/nu6051971. PMC 4042573. PMID 24841104.