Giant cell
A giant cell (multinucleated giant cell, multinucleate giant cell) is a mass formed by the union of several distinct cells (usually histiocytes), often forming a granuloma.[1] Although there is typically a focus on the pathological aspects of multinucleate giant cells (MGCs), they also play many important physiological roles. Osteoclasts specifically are invaluable to healthy physiological functions and are key players in the skeletal system. Osteoclasts are frequently classified and discussed separately from other MGCs which are more closely linked with human pathologies.
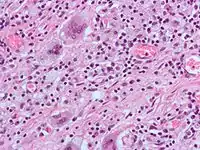
Non-osteoclast MGCs can arise in response to an infection, such as from tuberculosis, herpes, or HIV, or foreign body. These MGCs are cells of monocyte or macrophage lineage fused together. Similar to their monocyte precursors, they are able to phagocytose foreign materials. However, their large size and extensive membrane ruffling make them better equipped to clear up larger particles. They utilize activated CR3s to ingest complement-opsonized targets. Non-osteoclast MGCs are also responsible for the clearance of cell debris which is necessary for tissue remodeling after injuries.[2]
Types include foreign-body giant cell, Langhans giant cell, Touton giant cells, Giant-cell arteritis, and Reed–Sternberg cell.
History
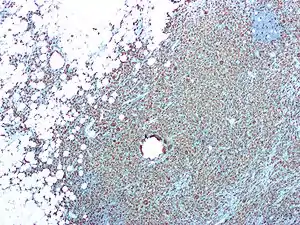
Osteoclasts were discovered in 1873.[3] However, it wasn't until the development of the organ culture in the 1970’s that their origin and function could be deduced. Although there was a general consensus early on about the physiological function of osteoclasts, theories on their origins were heavily debated. Many believed osteoclasts and osteoblasts came from the same progenitor cell. Because of this, osteoclasts were thought to be derived from cells in connective tissue. Studies that observed that bone resorption could be restored by bone marrow and spleen transplants helped prove osteoclasts hematopoietic origin.[3]
Other multinucleated giant cell formations can arise from numerous types of bacteria, diseases, and cell formations. Giant cells are known to develop when infections are also present. They were first noticed as early as the middle of the last century, but still it is not fully understood why these reactions occur. In the process of giant cell formation, monocytes or macrophages fuse together, which could cause multiple problems for the immune system.
Osteoclast
Osteoclasts are the most prominent examples of MGCs and are responsible for the resorption of bones in the body. Like other MGCs they are formed from the fusion of Monocyte/Macrophage precursors.[4] However, unlike other MGCs, the fusion pathway they originate from is well elucidated. They also do not ingest foreign materials and instead absorb bone matrix and minerals.
Osteoclasts are typically associated more with healthy physiological functions than they are to pathological states. They function alongside osteoblasts to remodel and maintain the integrity of bones in the body. They also contribute to the creation of the niche necessary for hematopoiesis, and negatively regulate T-cells. However, while Osteoclasts primary functions are integral to maintaining a healthy physiological state, they have also been linked to osteoporosis and the formation of bone tumors.[5]
Giant cell arteritis
The most common form of giant cell formations is giant-cell arteritis,[6] it is also known as temporal arteritis or cranial arteritis. This type of arteritis causes the arteries in the head, neck, and arm area to swell to abnormal sizes. Although the cause of this disease is not currently known, it does appear to be related with polymyalgia rheumatica.[7]
This disease is mostly known to affect older individuals that are in their fifties and sixties. Women are three times more likely to develop the disease than men, and Caucasians are seven times more likely to develop the disease than Africans.[8]
Symptoms
Symptoms may include a mild fever, loss of appetite, fatigue, vision loss, and severe headaches.[9] These symptoms are often misinterpreted leading to a delay in treatment.[10] If left untreated, this disease can result in permanent blindness.[11]
Diagnosis
The gold standard for diagnosis is a temporal artery biopsy.[12] The skin in the patient's face is anesthetised, and an incision is made in the face around the area of the temples to obtain a sample of the temporal artery. The incision will then be sutured. A histopathologist examines the sample under a microscope, who will then issue a pathology report (pending extra tests that may be requested by the pathologist).
The management regime consists primarily of systemic corticosteroids (e.g. prednisolone), commencing at a high dose.
Langhans giant cell
This particular form of giant cell was named after a German pathologist, Theodor Langhans. Like many of the other kinds of giant cell formations, the epithelioid macrophages fuse together, and in return multiple nuclei form. This formation is often referred to as multinucleated giant cells in which the nuclei form a circle or semicircle related to the shape of a horseshoe away from the center of the cell. Langhans giant cell is said to be related to tuberculosis and it occurs in many types of granulomatous diseases.
Who may be at risk
It is more common to get the disease if the person is infected with tuberculosis in endemic areas or develop sarcoidosis.
Symptoms
Langhans giant cell could be closely related to tuberculosis, syphilis, sarcoidosis and deep fungal infections. Langhans giant cell occurs frequently in delayed hypersensitivity.
Symptoms may include
- Fever
- Weight loss
- Fatigue
- Loss of appetite
Diagnosis
This type of giant cell could be caused by bacteria that spread from person to person through the air. Tuberculosis is related to HIV; many people who have HIV also have a hard time fighting off diseases and sicknesses. Many tests may be performed to treat other related diseases to obtain the correct diagnosis for Langhans giant cell.
Touton giant cell
This type of giant cell also is sometimes called a xanthelasmatic giant cell. Touton giant cells also consist of fused epithelioid macrophages and have multiple nuclei. The nuclei form a ring and are surrounded by foamy cytoplasm, one symptom of this particular giant cell is a foamy cytoplasm making the cytoplasm visible around the nucleus. This giant cell formation has been seen in lipid-laden or more known fat necrosis.
Who may be at risk
The formation of Touton giant cell is most common in men and women age 37 – 78.
Symptoms
Like all other forms of giant cells Touton giant cell has pretty much the same symptoms as any of the other form of giant cells. Which include:
- Fever
- Weight loss
- Fatigue
- Loss of appetite
Foreign-body giant cell
.jpg.webp)
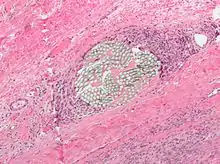
As the name implies, foreign-body giant cells form when a subject is exposed to a foreign substance. Exogenous substances include talc or sutures. As with other types of giant cells, epithelioid macrophages fusing together causes these giant cells to form and grow.[13] In this form of giant cell, the nuclei are arranged in an overlapping manner. This giant cell is often found in tissue because of medical devices, prostheses, and biomaterials.
Reed-Sternberg cell
These cells are generally thought to originate from B-lymphocytes.[14] However, because of how rare these cells are they are hard to study there are other theories about the origins of these cells. Some less popular theories speculate that they may arise from the fusion between reticulum cells, lymphocytes, and virus infected cells.[15]
Similar to other MGCs, Reed Sternberg cells are large and are either multinucleated or have a bilobed nucleus. Their nuclei are irregularly shaped, contain clear chromatin, and possess an eosinophilic nucleolus.[16]
Endogenous causative agents
Endogenous substances such as keratin, fat, and cholesterol crystals (cholesteatoma) can induce mast cell formation.[13]
Multinucleated giant cells in COVID-19 patients
Coronavirus disease 2019 ( COVID-19 ) is caused by a new coronavirus called SARS-CoV-2. Multinucleated giant cells have recently been detected in autopsy specimens from patients with COVID-19 disease. This type of giant cell was first found in pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer.[17] Another pathological study also detected this type of giant cell in COVID-19 and described it as “multinucleated syncytial cells”.[18] The morphological analysis showed that multinucleated syncytial cells with atypical enlarged pneumocytes characterized by large nuclei, amphophilic granular cytoplasm, and prominent nucleoli were identified in the intra-alveolar, spaces, showing viral cytopathic-like changes.[19] Interestingly, the viral antigen was detected in the cytoplasm of multinucleated syncytial cells.[20] The viral antigen detected in multinucleated giant cells indicates the presence of the SARS-CoV-2 virus. The infection and pathogenesis of the SARS-CoV-2 virus in the human patient largely remained unknown. A further study to characterize the role of multinucleated giant cells in human immune defense against COVID-19 may lead to more effective therapies.
See also
References
- Giant+Cells at the US National Library of Medicine Medical Subject Headings (MeSH)
- Milde, Ronny; Ritter, Julia; Tennent, Glenys A.; Loesch, Andrzej; Martinez, Fernando O.; Gordon, Siamon; Pepys, Mark B.; Verschoor, Admar; Helming, Laura (2015-11-25). "Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction". Cell Reports. 13 (9): 1937–1948. doi:10.1016/j.celrep.2015.10.065. ISSN 2211-1247. PMC 4675895. PMID 26628365.
- Martin, T. J. (2013). "Historically significant events in the discovery of RANK/RANKL/OPG". World Journal of Orthopedics. 4 (4): 186–197. doi:10.5312/wjo.v4.i4.186. PMC 3801238. PMID 24147254.
- Boyle, William J.; Simonet, W. Scott; Lacey, David L. (2003-05-15). "Osteoclast differentiation and activation". Nature. 423 (6937): 337–342. Bibcode:2003Natur.423..337B. doi:10.1038/nature01658. ISSN 0028-0836. PMID 12748652. S2CID 4428121.
- Charles, Julia F.; Aliprantis, Antonios O. (August 2014). "Osteoclasts: more than 'bone eaters'". Trends in Molecular Medicine. 20 (8): 449–459. doi:10.1016/j.molmed.2014.06.001. ISSN 1471-4914. PMC 4119859. PMID 25008556.
- "Giant Cell Arteritis: MedlinePlus". Nlm.nih.gov. Retrieved 2014-02-20.
- "Questions and Answers About Polymyalgia Rheumatica and Giant Cell Arteritis". Niams.nih.gov. Archived from the original on 2016-05-25. Retrieved 2014-02-20.
- Crowson, Cynthia S.; Matteson, Eric L. (October 2017). "Contemporary Prevalence Estimates for Giant Cell Arteritis and Polymyalgia Rheumatica, 2015". Seminars in Arthritis and Rheumatism. 47 (2): 253–256. doi:10.1016/j.semarthrit.2017.04.001. ISSN 0049-0172. PMC 5623160. PMID 28551169.
- Baig, Iyza F; Pascoe, Alexis R; Kini, Ashwini; Lee, Andrew G (2019-01-17). "Giant cell arteritis: early diagnosis is key". Eye and Brain. 11: 1–12. doi:10.2147/EB.S170388. ISSN 1179-2744. PMC 6340646. PMID 30697092.
- Ness, Thomas; Bley, Thorsten A; Schmidt, Wolfgang A; Lamprecht, Peter (May 2013). "The Diagnosis and Treatment of Giant Cell Arteritis". Deutsches Ärzteblatt International. 110 (21): 376–386. doi:10.3238/arztebl.2013.0376. ISSN 1866-0452. PMC 3679627. PMID 23795218.
- Singh, Abha G.; Kermani, Tanaz A.; Crowson, Cynthia S.; Weyand, Cornelia M.; Matteson, Eric L.; Warrington, Kenneth J. (February 2015). "Visual Manifestations in Giant Cell Arteritis: Trend over Five Decades in a Population-based Cohort". The Journal of Rheumatology. 42 (2): 309–315. doi:10.3899/jrheum.140188. ISSN 0315-162X. PMC 4367485. PMID 25512481.
- Giant Cell Arteritis (Temporal Arteritis) at eMedicine
- Saunders, William H.; Wakely Jr., Paul. "ATLAS OF HEAD AND NECK PATHOLOGY – Giant Cells" (PDF). Ohio State University Wexner Medical Center. Archived from the original (PDF) on 2014-04-16. Retrieved 15 Apr 2014.
- Steidl, Christian (5 January 2017). "Exposing Hodgkin-Reed-Sternberg cells". Blood. 129 (1): 6–7. doi:10.1182/blood-2016-11-746701. ISSN 1528-0020. PMID 28057670.
- Aggarwal, Payal; Limaiem, Faten (2020), "Reed Sternberg Cells", StatPearls, StatPearls Publishing, PMID 31194473, retrieved 2020-04-29
- Aggarwal, Payal; Limaiem, Faten (2020), "Reed Sternberg Cells", StatPearls, StatPearls Publishing, PMID 31194473, retrieved 2020-05-01
- 17
- 18
- 18
- 19