Kupffer cell
Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells contain the largest amount of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis.[2][3] They form part of the mononuclear phagocyte system.
| Kupffer cell | |
|---|---|
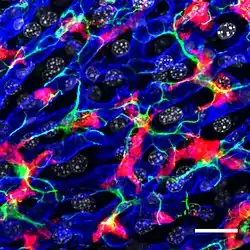 Confocal microscopy picture showing the steady-state location and interactions between Kupffer cells (Red), hepatic stellate cells (green) and liver sinusoidal endothelial cells (blue). Cell nuclei are in grey.[1] | |
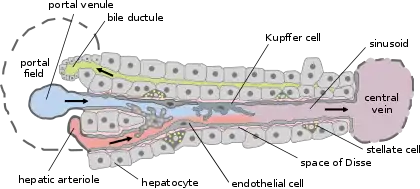 Basic liver structure | |
| Details | |
| Location | Liver |
| Function | Macrophage |
| Identifiers | |
| Latin | macrophagocytus stellatus |
| MeSH | D007728 |
| TH | H3.04.05.0.00016 |
| FMA | 14656 |
| Anatomical terms of microanatomy | |
Structure
The amoeboid shaped Kupffer cells are attached to sinusoidal endothelial cells. The surface of Kupffer cells contain microvilli, pseudopodia and lamellipodia, which project in every direction. The microvilli and pseudopodia play a role in the endocytosis of particles. In their cytoplasm can be found ribosomes, Golgi apparatus, centrioles, microtubules and microfilaments. The nucleus is indented and ovoid and can be lobulated. They also contain rough endoplasmic reticulum, nuclear envelope, and annulate lamellae, which all contain peroxidase activity. Both the centrilobular and periportal regions of the liver, house Kupffer cells, but their function and structures change depending on their location. Periportal Kupffer cells tend to be larger and have more lysosomal enzyme and phagocytic activity, whereas centrilobular Kupffer cells create more superoxide radical. The SR-AI/II scavenger receptor is found inside Kupffer cells. This receptor is involved in recognising and binding the lipid A domain of lipopolysaccharide (LPS) and lipoteichoic acid. Lipopolysaccharide (LPS) is a bacterial endotoxin which is found in the cell wall gram-negative bacteria, whereas lipoteichoic acid is present in gram-positive bacteria.[4]
Development
Their development begins in the yolk sac where they differentiate into fetal macrophages. Once they enter the blood stream, they migrate to the fetal liver where they stay. There they complete their differentiation into Kupffer cells.[5]
There are mainly two types of hepatic macrophages: Kupffer cells, that reside in the liver and originated from yolk sac-derived red bone marrow progenitor cells, and also monocyte-derived macrophages, derived from hematopoietic stem cells from the bone marrow and transported through the blood circulation to the liver. Monocyte-derived macrophages are immunogenic macrophages that differentiate when under the influence of microenvironment. Kupffer cells self-sustain. They locally proliferate, they are usually a susceptible population of phagocytic cells. Monocytes that are in the peripheral circulation and come from precursor cells in the bone marrow, are thought to be immature precursors for tissue macrophages, in adult animals. The liver can be entered by peripheral blood monocytes, then the monocytes mature into a phenotype characteristic of tissue macrophages. Various growth factors regulate the differentiation of macrophages. However, the role of stimulating macrophage colony seems to be the most important in the developing of mature Kupffer cells.[6][3]
Function
The average life of a Kupffer cell is around 3.8 days. The primary function of the Kupffer cell is to remove foreign debris and particles that have comes from the portal system when passing through the liver. It is possible for the Kupffer cells to take in large particles by phagocytosis and smaller particles via pinocytosis.[4] Kupffer cells are integral in the innate responses of the immune system. They are also important for host defense and play a role in the metabolism of many different compounds including, lipids, protein complexes and small particles. They are also useful in removing apoptotic cells from circulation.[2][3] The amount of Kupffer cells is constant in the liver. They are regulated by apoptosis, as well as being phagocytized by neighbouring Kupffer cells. Kupffer cells have a proliferative capacity, this allows them to regenerate themselves, this is in complete contrast to monocyte-derived macrophages that have no proliferative potential. Kupffer cells have shown to be heterogeneous in their function, dependent on their location in the body. For example, in zone 1 of the liver lobules, they are much more active than their counterparts in zone 3. The heightened activity can be attributed to there being much more exposure to dangerous substances in zone 1 than in zone 3. Kupffer cells can produce inflammatory cytokines, TNF-alpha, oxygen radicals, and proteases as well as performing phagocytosis. Creating these mediators is believed to lead to the development of liver injury.[4]
Apart from clearing any bacteria, red blood cells are also broken down by phagocytic action, where the hemoglobin molecule is split. The globin chains are re-used, while the iron-containing portion, heme, is further broken down into iron, which is re-used, and bilirubin, which is conjugated to glucuronic acid within hepatocytes and secreted into the bile.
Helmy et al. identified a receptor present in Kupffer cells, the complement receptor of the immunoglobulin family (CRIg). Mice without CRIg could not clear complement system-coated pathogens. CRIg is conserved in mice and humans and is a critical component of the innate immune system.[7]
Clinical significance
Kupffer cell activation is responsible for early ethanol-induced liver injury, common in chronic alcoholics. Chronic alcoholism and liver injury deal with a two hit system. The second hit is characterized by an activation of the Toll-like receptor 4 (TLR4) and CD14, receptors on the Kupffer cell that internalize endotoxin (lipopolysaccharide or LPS). This activates the transcription of pro-inflammatory cytokines (Tumor necrosis factor-alpha or TNFα) and production of superoxides (a pro-oxidant). TNFα will then enter the stellate cell in the liver, leading to collagen synthesis and fibrosis. Fibrosis will eventually cause cirrhosis, or loss of function of the liver.[8]
In response to sepsis, Kupffer cells play a role in the pathogenesis of a damaged liver. The macrophages in the liver activate and release both IL-1 and TNF-alpha. In turn, this activates leukocytes and sinusoidal endothelial cells to express ICAM-1. This results in tissue damage to the endothelium because of proteases, oxygen radicals, prostanoids and other substances from leukocytes. Alcoholic liver disease, or ALD as it is known, is a term that includes a wide range of liver injuries from steatosis to steatotohepatitis, fibrosis and cirrhosis. Evidence has shown that Kupffer cells play an important role in pathogenesis of both chronic and acute ALD. Exposure to alcohol can result in increased hepatic translocation endotoxin/lipopolysacharide sourced from the gut, which is a strong M1 polarization of Kupffer cells. A large amount of reactive oxygen species, pro-inflammatory cytokines and chemokines are produced by the activated Kupffer cells which lead to liver injury. Kupffer cells are incredibly plastic cells that have the capability to polarize specific activation states and can perform different functions in different microenvironments. M1 (classical activation) and M2 (alternative activation) are the two designation of the two extremes of macrophage polarization. M1 polarized Kupffer produces a large amount of pro-inflammatory cytokines like TNF-alpha. On the other hand, M2-polarized Kupffer cells demonstrate a large quantity of anti-inflammatory mediators, for example, IL-10.[9][4]
History
The cells were first observed by Karl Wilhelm von Kupffer in 1876.[10] The scientist called them "Sternzellen" (star cells or hepatic stellate cell) but thought, inaccurately, that they were an integral part of the endothelium of the liver blood vessels and that they originated from it. In 1898, after several years of research, Tadeusz Browicz identified them, correctly, as macrophages.[11][12]
References
- Bonnardel J, T'Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, et al. (October 2019). "Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche". Immunity. 51 (4): 638–654.e9. doi:10.1016/j.immuni.2019.08.017. PMC 6876284. PMID 31561945.
- Nguyen-Lefebvre, Anh Thu; Horuzsko, Anatolij (2015). "Kupffer Cell Metabolism and Function". Journal of Enzymology and Metabolism. 1 (1). PMC 4771376. PMID 26937490.
- Dixon, Laura J.; Barnes, Mark; Tang, Hui; Pritchard, Michele T.; Nagy, Laura E. (April 2013). "Kupffer Cells in the Liver". Comprehensive Physiology. 3 (2): 785–797. doi:10.1002/cphy.c120026. ISSN 2040-4603. PMC 4748178. PMID 23720329.
- Basit, Hajira; Tan, Michael L.; Webster, Daniel R. (2020), "Histology, Kupffer Cell", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29630278, retrieved 2020-08-25
- Naito M, Hasegawa G, Takahashi K (November 1997). "Development, differentiation, and maturation of Kupffer cells". Microscopy Research and Technique. 39 (4): 350–64. doi:10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L. PMID 9407545.
- "Kupffer Cells in Non-alcoholic Fatty Liver Disease: Friend or Foe?". www.ijbs.com. Retrieved 2020-08-31.
- Helmy KY, Katschke KJ, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, et al. (March 2006). "CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens". Cell. 124 (5): 915–27. doi:10.1016/j.cell.2005.12.039. PMID 16530040. S2CID 15525209.
- Wheeler MD (2003). "Endotoxin and Kupffer cell activation in alcoholic liver disease". Alcohol Research & Health : The Journal of the National Institute on Alcohol Abuse and Alcoholism. 27 (4): 300–6. PMC 6668869. PMID 15540801.
- Zeng, Tao; Zhang, Cui-Li; Xiao, Mo; Yang, Rui; Xie, Ke-Qin (2016). "Critical Roles of Kupffer Cells in the Pathogenesis of Alcoholic Liver Disease: From Basic Science to Clinical Trials". Frontiers in Immunology. 7: 538. doi:10.3389/fimmu.2016.00538. ISSN 1664-3224. PMC 5126119. PMID 27965666.
- Haubrich WS (July 2004). "Kupffer of Kupffer cells". Gastroenterology. 127 (1): 16. doi:10.1053/j.gastro.2004.05.041. PMID 15236167.
- Szymańska R, Schmidt-Pospuła M (1979). "[Studies of liver's reticuloendothelial cells by Tadeusz Browicz and Karl Kupffer. A historical outline]". Archiwum Historii Medycyny. 42 (3): 331–6. PMID 386989.
- Stachura J, Gałazka K (December 2003). "History and current status of Polish gastroenterological pathology". Journal of Physiology and Pharmacology. 54 Suppl 3: 183–92. PMID 15075472.
External links
- Anatomy photo: digestive/mammal/liver5/liver4 - Comparative Organology at University of California, Davis - "Mammal, liver (EM, Low)"
- Histology image: 15508loa – Histology Learning System at Boston University
- Kupffer Cell Foundation - The mission of the Kupffer cell Foundation is to stimulate and support research and education to improve knowledge on the role of the Kupffer cell and sinusoidal barrier in healthy and diseased liver