Mechanical ventilation
Mechanical ventilation, assisted ventilation or intermittent mandatory ventilation (IMV), is the medical term for artificial ventilation where mechanical means are used to assist or replace spontaneous breathing.[1] This may involve a machine called a ventilator, or the breathing may be assisted manually by a suitably qualified professional, such as an anesthesiologist, registered nurse (RN), paramedic or other first responder, or in some parts of the United States, by a respiratory therapist (RT), by compressing a bag valve mask device.
| Mechanical ventilation | |
|---|---|
| ICD-9 | 93.90 96.7 |
| MeSH | D012121 |
| OPS-301 code | 8-71 |
Mechanical ventilation is termed "invasive" if it involves any instrument inside the trachea through the mouth, such as an endotracheal tube or the skin, such as a tracheostomy tube. [2] Face or nasal masks are used for non-invasive ventilation in appropriately selected conscious patients.
The two main types of mechanical ventilation include positive pressure ventilation where air (or another gas mix) is pushed into the lungs through the airways, and negative pressure ventilation where air is usually, in essence, sucked into the lungs by stimulating movement of the chest. Apart from these two main types, there are many specific modes of mechanical ventilation, and their nomenclature has been revised over the decades as the technology has continually developed.
Uses

Mechanical ventilation is indicated when the patient's spontaneous breathing is inadequate to maintain life. It is also indicated as prophylaxis for imminent collapse of other physiologic functions, or ineffective gas exchange in the lungs. Because mechanical ventilation serves only to provide assistance for breathing and does not cure a disease, the patient's underlying condition should be identified and treated in order to resolve over time. In addition, other factors must be taken into consideration because mechanical ventilation is not without its complications [3]
In general, mechanical ventilation is initiated to protect the airway/reduce work of breathing and/or correct blood gases.
Common specific medical indications for use include:
- Acute lung injury, including acute respiratory distress syndrome (ARDS) and trauma
- Apnea with respiratory arrest, including cases from intoxication
- Acute severe asthma requiring intubation
- Acute or chronic respiratory acidosis, most commonly with chronic obstructive pulmonary disease (COPD) and obesity hypoventilation syndrome
- Acute respiratory acidosis with partial pressure of carbon dioxide (pCO
2) > 50 mmHg and pH < 7.25, which may be due to paralysis of the diaphragm due to Guillain–Barré syndrome, myasthenia gravis, motor neuron disease, spinal cord injury, or the effect of anaesthetics and muscle relaxants - Increased work of breathing as evidenced by significant tachypnea, retractions, and other physical signs of respiratory distress[4]
- Hypoxemia with arterial partial pressure of oxygen (PaO
2) < 55 mm Hg with supplemental fraction of inspired oxygen (FiO
2) = 1.0 - Hypotension including sepsis, shock, congestive heart failure
- Neurological diseases such as muscular dystrophy and amyotrophic lateral sclerosis (ALS)
Risk
Mechanical ventilation is often a life-saving intervention, but carries potential complications including pneumothorax, airway injury, alveolar damage, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis.[5][6] Other complications include diaphragm atrophy, decreased cardiac output, and oxygen toxicity. One of the primary complications that presents in patients mechanically ventilated is acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). ALI/ARDS are recognized as significant contributors to patient morbidity and mortality.[7]
In many healthcare systems, prolonged ventilation as part of intensive care is a limited resource (in that there are only so many patients that can receive care at any given moment). It is used to support a single failing organ system (the lungs) and cannot reverse any underlying disease process (such as terminal cancer). For this reason, there can be (occasionally difficult) decisions to be made about whether it is suitable to commence someone on mechanical ventilation. Equally many ethical issues surround the decision to discontinue mechanical ventilation.[8]
- Pulmonary barotrauma is a well-known complication of positive-pressure mechanical ventilation.[9] This includes pneumothorax, subcutaneous emphysema, pneumomediastinum, and pneumoperitoneum.[9]
- Ventilator-associated lung injury (VALI) refers to acute lung injury that occurs during mechanical ventilation. It is clinically indistinguishable from acute lung injury or acute respiratory distress syndrome (ALI/ARDS).[10]
- Diaphragm disuse atrophy may result from controlled mechanical ventilation, a rapid type of atrophy involving the diaphragmatic muscle fibers that can develop in the first day of mechanical ventilation.[11] This cause of atrophy in the diaphragm is also a cause of atrophy in all respiratory related muscles during controlled mechanical ventilation.[12]
- Impaired mucociliary motility in the airways may result from positive pressure ventilation; bronchial mucus transport is frequently impaired, and is associated with retention of secretions, and pneumonia.[13]
Theory
In theory, the transairway pressure is simply the difference between the pressure at airway opening and the pressure in the alveoli.[14] That is,
where PTA is the transairway pressure, PAO is the pressure at the airway opening, and PALV is the pressure in the alveoli.
The following are further formulas to calculate various parameters related to ventilation.
- Alveolar ventilation:
- Arterial PaCO2:
- Alveolar volume:
- Estimated physiologic shunt equation:
When 100% oxygen (1.00 FiO
2) is used initially for an adult, it is easy to calculate the next FiO
2 to be used, and easy to estimate the shunt fraction. The estimated shunt fraction refers to the amount of oxygen not being absorbed into the circulation. In normal physiology, gas exchange (oxygen/carbon dioxide) occurs at the level of the alveoli in the lungs. The existence of a shunt refers to any process that hinders this gas exchange, leading to wasted oxygen inspired and the flow of un-oxygenated blood back to the "left heart" (which ultimately supplies the rest of the body with unoxygenated blood). When using 100% oxygen, the degree of shunting is estimated as:
- 700 mmHg — measured PaO
2 (from an arterial blood gas)
For each difference of 100 mmHg, the shunt is 5%. A shunt of more than 25% should prompt a search for the cause of this hypoxemia, such as mainstem intubation or pneumothorax, and should be treated accordingly. If such complications are not present, other causes must be sought after, and positive end-expiratory pressure (PEEP) should be used to treat this intrapulmonary shunt. Other such causes of a shunt include:
- alveolar collapse from major atelectasis; and
- alveolar collection of material other than gas, such as pus from pneumonia, water and protein from acute respiratory distress syndrome, water from congestive heart failure, or blood from haemorrhage.
Mechanical dead space is a further important parameter in ventilator design and function. It is defined as the volume of gas breathed again as the result of use in a mechanical device. The following is an example calculation for mechanical dead space.
A simplified version of the same is:
Application and duration
It can be used as a short-term measure, for example during an operation or critical illness (often in the setting of an intensive-care unit). It may be used at home or in a nursing or rehabilitation institution if patients have chronic illnesses that require long-term ventilatory assistance. Due to the anatomy of the human pharynx, larynx, and esophagus and the circumstances for which ventilation is needed, additional measures are often required to secure the airway during positive-pressure ventilation in order to allow unimpeded passage of air into the trachea and avoid air passing into the esophagus and stomach. The common method is by insertion of a tube into the trachea: intubation, which provides a clear route for the air. This can be either an endotracheal tube, inserted through the natural openings of mouth or nose, or a tracheostomy inserted through an artificial opening in the neck. In other circumstances simple airway maneuvres, an oropharyngeal airway or laryngeal mask airway may be employed. If the patient is able to protect his/her own airway and non-invasive ventilation or negative-pressure ventilation is used then an airway adjunct may not be needed.
Types of ventilators

Ventilators come in many different styles and method of giving a breath to sustain life. There are manual ventilators such as bag valve masks and anesthesia bags that require the users to hold the ventilator to the face or to an artificial airway and maintain breaths with their hands. Mechanical ventilators are ventilators not requiring operator effort and are typically computer-controlled or pneumatic-controlled. Mechanical ventilators typically require power by a battery or a wall outlet (DC or AC) though some ventilators work on a pneumatic system not requiring power. There are a variety of technologies available for ventilation, falling into two main (and then lesser categories), the two being the older technology of negative-pressure mechanisms, and the more common positive-pressure types.
Common positive-pressure mechanical ventilators include:
- Transport ventilators—These ventilators are small and more rugged, and can be powered pneumatically or via AC or DC power sources.
- Intensive-care ventilators—These ventilators are larger and usually run on AC power (though virtually all contain a battery to facilitate intra-facility transport and as a back-up in the event of a power failure). This style of ventilator often provides greater control of a wide variety of ventilation parameters (such as inspiratory rise time). Many ICU ventilators also incorporate graphics to provide visual feedback of each breath.
- Neonatal ventilators (bubble CPAP)—Designed with the preterm neonate in mind, these are a specialized subset of ICU ventilators that are designed to deliver the smaller, more precise volumes and pressures required to ventilate these patients.
- Positive airway pressure ventilators (PAP) — These ventilators are specifically designed for non-invasive ventilation. This includes ventilators for use at home for treatment of chronic conditions such as sleep apnea or COPD.
Modes of mechanical ventilation
Mechanical ventilation utilizes several separate systems for ventilation referred to as the mode. Modes come in many different delivery concepts but all modes fall into one of three categories; volume-cycled, pressure-cycled, spontaneously cycled. In general, the selection of which mode of mechanical ventilation to use for a given patient is based on the familiarity of clinicians with modes and the equipment availability at a particular institution.[15]
Positive pressure

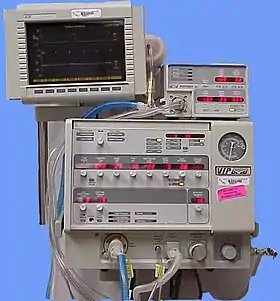
The design of the modern positive-pressure ventilators were based mainly on technical developments by the military during World War II to supply oxygen to fighter pilots in high altitude. Such ventilators replaced the iron lungs as safe endotracheal tubes with high-volume/low-pressure cuffs were developed. The popularity of positive-pressure ventilators rose during the polio epidemic in the 1950s in Scandinavia[16][17] and the United States and was the beginning of modern ventilation therapy. Positive pressure through manual supply of 50% oxygen through a tracheostomy tube led to a reduced mortality rate among patients with polio and respiratory paralysis. However, because of the sheer amount of man-power required for such manual intervention, mechanical positive-pressure ventilators became increasingly popular.
Positive-pressure ventilators work by increasing the patient's airway pressure through an endotracheal or tracheostomy tube. The positive pressure allows air to flow into the airway until the ventilator breath is terminated. Then, the airway pressure drops to zero, and the elastic recoil of the chest wall and lungs push the tidal volume — the breath-out through passive exhalation.
Negative pressure machines
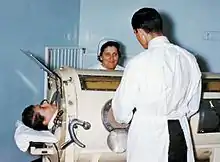
Negative pressure mechanical ventilators are produced in small, field-type and larger formats. The prominent design of the smaller devices is known as the cuirass, a shell-like unit used to create negative pressure only to the chest using a combination of a fitting shell and a soft bladder.[lower-alpha 1] In recent years this device has been manufactured using various-sized polycarbonate shells with multiple seals, and a high-pressure oscillation pump in order to carry out biphasic cuirass ventilation. Its main use has been in patients with neuromuscular disorders that have some residual muscular function. The latter, larger formats are in use, notably with the polio wing hospitals in England such as St Thomas' Hospital in London and the John Radcliffe in Oxford.
The larger units have their origin in the iron lung, also known as the Drinker and Shaw tank, which was developed in 1929 and was one of the first negative-pressure machines used for long-term ventilation. It was refined and used in the 20th century largely as a result of the polio epidemic that struck the world in the 1940s. The machine is, in effect, a large elongated tank, which encases the patient up to the neck. The neck is sealed with a rubber gasket so that the patient's face (and airway) are exposed to the room air. While the exchange of oxygen and carbon dioxide between the bloodstream and the pulmonary airspace works by diffusion and requires no external work, air must be moved into and out of the lungs to make it available to the gas exchange process. In spontaneous breathing, a negative pressure is created in the pleural cavity by the muscles of respiration, and the resulting gradient between the atmospheric pressure and the pressure inside the thorax generates a flow of air. In the iron lung by means of a pump, the air is withdrawn mechanically to produce a vacuum inside the tank, thus creating negative pressure. This negative pressure leads to expansion of the chest, which causes a decrease in intrapulmonary pressure, and increases flow of ambient air into the lungs. As the vacuum is released, the pressure inside the tank equalizes to that of the ambient pressure, and the elastic coil of the chest and lungs leads to passive exhalation. However, when the vacuum is created, the abdomen also expands along with the lung, cutting off venous flow back to the heart, leading to pooling of venous blood in the lower extremities. There are large portholes for nurse or home assistant access. The patients can talk and eat normally, and can see the world through a well-placed series of mirrors. Some could remain in these iron lungs for years at a time quite successfully.
Intermittent abdominal pressure ventilator
Another type is the intermittent abdominal pressure ventilator that applies pressure externally via an inflated bladder, forcing exhalation, sometimes termed exsufflation. The first such apparatus was the Bragg-Paul Pulsator.[18][19] The name of one such device, the Pneumobelt made by Puritan Bennett has to a degree become a generic name for the type.[19][20]
Trigger
The trigger is what causes a breath to be delivered by a mechanical ventilator. Breaths may be triggered by a patient taking their own breath, a ventilator operator pressing a manual breath button, or by the ventilator based on the set breath rate and mode of ventilation.
Cycle
The cycle is what causes the breath to transition from the inspiratory phase to the exhalation phase. Breaths may be cycled by a mechanical ventilator when a set time has been reached, or when a preset flow or percentage of the maximum flow delivered during a breath is reached depending on the breath type and the settings. Breaths can also be cycled when an alarm condition such as a high pressure limit has been reached, which is a primary strategy in pressure regulated volume control.
Limit
Limit is how the breath is controlled. Breaths may be limited to a set maximum circuit pressure or a set maximum flow.
Breath exhalation
Exhalation in mechanical ventilation is almost always completely passive. The ventilator's expiratory valve is opened, and expiratory flow is allowed until the baseline pressure (PEEP) is reached. Expiratory flow is determined by patient factors such as compliance and resistance.
Weaning from mechanical ventilation
Timing of withdrawal from mechanical ventilation—also known as weaning—should be carefully considered. Patients should have their ventilation considered for withdrawal if they are able to support their own ventilation and oxygenation, and this should be assessed continuously. There are several objective parameters to look for when considering withdrawal, but there are no specific criteria that generalizes to all patients.
The Rapid Shallow Breathing Index (RSBI, the ratio of respiratory frequency to tidal volume (f/VT), previously referred to as the "Tobin Index" after Dr. Martin Tobin of Loyola University Medical Center) is one of the best studied and most commonly used weaning predictors, with no other predictor having been shown to be superior. It was described in a prospective cohort study of mechanically ventilated patients which found that a RSBI > 105 breaths/min/L was associated with weaning failure, while a RSBI < 105 breaths/min/L predicted weaning success with a sensitivity, specificity, positive predictive value and negative predictive value of 97%, 64%, 78%, 95% respectively.[21]
Respiratory monitoring

One of the main reasons why a patient is admitted to an ICU is for delivery of mechanical ventilation. Monitoring a patient in mechanical ventilation has many clinical applications: Enhance understanding of pathophysiology, aid with diagnosis, guide patient management, avoid complications and assessment of trends.[22]
In ventilated patients, pulse oximetry it is commonly used when titrating FIO2. A reliable target of Spo2 is greater than 95%.[23]
Different strategies exist to find the level of PEEP in these patients with ARDS[24] guided by esophageal pressure,[25] Stress Index,[26] static airway pressure-volume curve.[27] In such patients, some experts recommend limiting PEEP to low levels (~10cmH2O). In patients who have diffused loss of aeration, PEEP can be used provided it does not cause the plateau pressure to rise above the upper inflection point.
Most modern ventilators have basic monitoring tools. There are also monitors that work independently of the ventilator which allow for measuring patients after the ventilator has been removed, such as a T tube test.
Artificial airways as a connection to the ventilator
There are various procedures and mechanical devices that provide protection against airway collapse, air leakage, and aspiration:
- Face mask — In resuscitation and for minor procedures under anaesthesia, a face mask is often sufficient to achieve a seal against air leakage. Airway patency of the unconscious patient is maintained either by manipulation of the jaw or by the use of nasopharyngeal or oropharyngeal airway. These are designed to provide a passage of air to the pharynx through the nose or mouth, respectively. Poorly fitted masks often cause nasal bridge ulcers, a problem for some patients. Face masks are also used for non-invasive ventilation in conscious patients. A full-face mask does not, however, provide protection against aspiration. Non-invasive ventilation can be considered for epidemics of COVID-19 where sufficient invasive ventilation capacity is not available (or in some milder cases) but pressurized protection suits for caregivers are recommended due to the risks of poorly fitting masks emitting contaminating aerosols.[28]
- Tracheal intubation is often performed for mechanical ventilation of hours to weeks duration. A tube is inserted through the nose (nasotracheal intubation) or mouth (orotracheal intubation) and advanced into the trachea. In most cases, tubes with inflatable cuffs are used for protection against leakage and aspiration. Intubation with a cuffed tube is thought to provide the best protection against aspiration. Tracheal tubes inevitably cause pain and coughing. Therefore, unless a patient is unconscious or anaesthetized for other reasons, sedative drugs are usually given to provide tolerance of the tube. Other disadvantages of tracheal intubation include damage to the mucosal lining of the nasopharynx or oropharynx and subglottic stenosis.
- Supraglottic airway — a supraglottic airway (SGA) is any airway device that is seated above and outside the trachea, as an alternative to endotracheal intubation. Most devices work via masks or cuffs that inflate to isolate the trachea for oxygen delivery. Newer devices feature esophageal ports for suctioning or ports for tube exchange to allow intubation. Supraglottic airways differ primarily from tracheal intubation in that they do not prevent aspiration. After the introduction of the laryngeal mask airway (LMA) in 1998, supraglottic airway devices have become mainstream in both elective and emergency anesthesia.[29] There are many types of SGAs available including the esophageal-tracheal combitube (ETC), laryngeal tube (LT), and the obsolete esophageal obturator airway (EOA).
- Cricothyrotomy — Patients requiring emergency airway management, in whom tracheal intubation has been unsuccessful, may require an airway inserted through a surgical opening in the cricothyroid membrane. This is similar to a tracheostomy but a cricothyrotomy is reserved for emergency access.[30]
- Tracheostomy — When patients require mechanical ventilation for several weeks, a tracheostomy may provide the most suitable access to the trachea. A tracheostomy is a surgically created passage into the trachea. Tracheostomy tubes are well tolerated and often do not necessitate any use of sedative drugs. Tracheostomy tubes may be inserted early during treatment in patients with pre-existing severe respiratory disease, or in any patient expected to be difficult to wean from mechanical ventilation, i.e., patients with little muscular reserve.
- Mouthpiece — Less common interface, does not provide protection against aspiration. There are lipseal mouthpieces with flanges to help hold them in place if patient is unable.
History
The Greek physician Galen may have been the first to describe mechanical ventilation: "If you take a dead animal and blow air through its larynx [through a reed], you will fill its bronchi and watch its lungs attain the greatest distention."[31] Vesalius too describes ventilation by inserting a reed or cane into the trachea of animals.[32] In 1908 George Poe demonstrated his mechanical respirator by asphyxiating dogs and seemingly bringing them back to life.[33]
See also
- Biotrauma
- Charles Hederer, inventor of the pulmoventilateur
- Ventilator – Device that provides mechanical ventilation to the lungs
- Extracorporeal membrane oxygenation – Technique of providing both cardiac and respiratory support
Notes
- The early types of these were prone to falling off and caused severe chafing, and so were not used as a long-term devices.
References
- "What Is a Ventilator?". National Heart, Lung, and Blood Institute, National Instititutes of Health. Retrieved 2016-03-27.
- GN-13: Guidance on the Risk Classification of General Medical Devices Archived 2014-05-29 at the Wayback Machine, Revision 1.1. From Health Sciences Authority. May 2014
- "Ventilator Management: Introduction to Ventilator Management, Modes of Mechanical Ventilation, Methods of Ventilatory Support". 2020-04-07. Cite journal requires
|journal=(help) - Tulaimat, A; Patel, A; Wisniewski, M; Gueret, R (August 2016). "The validity and reliability of the clinical assessment of increased work of breathing in acutely ill patients". Journal of Critical Care. 34: 111–5. doi:10.1016/j.jcrc.2016.04.013. PMID 27288621.
- Hess DR (2011). "Approaches to conventional mechanical ventilation of the patient with acute respiratory distress syndrome". Respir Care. 56 (10): 1555–72. doi:10.4187/respcare.01387. PMID 22008397.
- Craven, DE; Chroneou, A; Zias, N; Hjalmarson, KI (February 2009). "Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes". Chest. 135 (2): 521–528. doi:10.1378/chest.08-1617. PMID 18812452.
- Hoesch, Robert; Eric Lin; Mark Young; Rebecca Gottesman; Laith Altaweel; Paul Nyquist; Robert Stevens (February 2012). "Acute lung injury in critical neurological illness". Critical Care Medicine. 40 (2): 587–593. doi:10.1097/CCM.0b013e3182329617. PMID 21946655. S2CID 9038265.
- O'Connor HH (2011). "Prolonged mechanical ventilation: are you a lumper or a splitter?". Respir Care. 56 (11): 1859–60. doi:10.4187/respcare.01600. PMID 22035828.
- Parker JC, Hernandez LA, Peevy KJ (1993). "Mechanisms of ventilator-induced lung injury". Crit Care Med. 21 (1): 131–43. doi:10.1097/00003246-199301000-00024. PMID 8420720. S2CID 23200644.
- "International consensus conferences in intensive care medicine: Ventilator-associated Lung Injury in ARDS". Am. J. Respir. Crit. Care Med. 160 (6): 2118–24. December 1999. doi:10.1164/ajrccm.160.6.ats16060. PMID 10588637.
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. (2008). "Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans". N Engl J Med. 358 (13): 1327–35. doi:10.1056/NEJMoa070447. PMID 18367735.
- De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. (2002). "Paresis acquired in the intensive care unit: a prospective multicenter study". JAMA. 288 (22): 2859–67. doi:10.1001/jama.288.22.2859. PMID 12472328.
- Konrad F, Schreiber T, Brecht-Kraus D, Georgieff M (1994). "Mucociliary transport in ICU patients". Chest. 105 (1): 237–41. doi:10.1378/chest.105.1.237. PMID 8275739.
- "Physiology: Respiratory". www.kumc.edu. Retrieved 2020-05-01.
- Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, et al. (2000). "How is mechanical ventilation employed in the intensive care unit? An international utilization review". Am J Respir Crit Care Med. 161 (5): 1450–8. doi:10.1164/ajrccm.161.5.9902018. PMID 10806138.
- Engstrom, C.-G. (1954). "Treatment of Severe Cases of Respiratory Paralysis by the Engstrom Universal Respirator". BMJ. 2 (4889): 666–669. doi:10.1136/bmj.2.4889.666. ISSN 0959-8138. PMC 2079443. PMID 13190223.
- US US2699163A, Engström, Carl Gunnar, "Respirator", issued 1951-06-25
- Bach, John Robert; Alba, A S (April 1991). "Intermittent abdominal pressure ventilator in a regimen of noninvasive ventilatory support". Chest. 99 (3): 630–6. doi:10.1378/chest.99.3.630. PMID 1899821. Retrieved 11 October 2016.
- Gilgoff, Irene S. (2001). Breath of Life: The Role of the Ventilator in Managing Life-Threatening Illnesses. Scarecrow Press. p. 187. ISBN 9780810834880. Retrieved 11 October 2016.
- Mosby's Medical Dictionary (8 ed.). 2009. Retrieved 11 October 2016.
- Yang KL, Tobin MJ (May 1991). "A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation". N. Engl. J. Med. 324 (21): 1445–50. doi:10.1056/NEJM199105233242101. PMID 2023603.
- Tobin, Martin J. (2012). Principles And Practice of Mechanical Ventilation (3rd ed.). McGraw Hill. ISBN 978-0-07-176678-4.
- Jubran A, Tobin MJ (1990). "Reliability of pulse oximietry in titrating supplemental oxygen therapy in ventilator dependant patients". Chest. 97 (6): 1420–5. doi:10.1378/chest.97.6.1420. PMID 2347228.
- Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, et al. (Acute Respiratory Distress Syndrome Network) (2000). "Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome". N Engl J Med. 342 (18): 1301–8. doi:10.1056/NEJM200005043421801. PMID 10793162.
- Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH (2008). "Mechanical ventilation guided by esophageal pressure in acute lung injury". N Engl J Med. 359 (20): 2095–104. doi:10.1056/NEJMoa0708638. PMC 3969885. PMID 19001507.
- Grasso S, Stripoli T, De Michele M, Bruno F, Moschetta M, Angelelli G, Munno I, Ruggiero V, Anaclerio R, Cafarelli A, Driessen B, Fiore T (2007). "ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure". Am J Respir Crit Care Med. 176 (8): 761–7. doi:10.1164/rccm.200702-193OC. PMID 17656676.
- Brochard, L. (1998). "Respiratory pressure-volume curves". In Tobin, M.J. (ed.). Principles and practice of intensive care monitoring. McGraw-Hill. pp. 597–616. ISBN 9780070650947.
- Murthy S, Gomersall CD, Fowler RA (2020-03-11). "Care for Critically Ill Patients With COVID-19". JAMA. 323 (15): 1499–1500. doi:10.1001/jama.2020.3633. PMID 32159735.
- Cook T, Howes B (December 2011). "Supraglottic airway devices: recent advances". Contin Educ Anaesth Crit Care. 11 (2): 56–61. doi:10.1093/bjaceaccp/mkq058.
- Carley SD, Gwinnutt C, Butler J, Sammy I, Driscoll P (March 2002). "Rapid sequence induction in the emergency department: a strategy for failure". Emerg Med J. 19 (2): 109–13. doi:10.1136/emj.19.2.109. PMC 1725832. PMID 11904254.
- Colice, Gene L (2006). "Historical Perspective on the Development of Mechanical Ventilation". In Martin J Tobin (ed.). Principles & Practice of Mechanical Ventilation (2 ed.). New York: McGraw-Hill. ISBN 978-0-07-144767-6.
- Chamberlain D (2003). "Never quite there: a tale of resuscitation medicine". Clin Med. 3 (6): 573–7. doi:10.7861/clinmedicine.3-6-573. PMC 4952587. PMID 14703040.
- "Smother Small Dog To See it Revived". New York Times. May 29, 1908. Retrieved 2007-12-25.
Successful Demonstration of an Artificial Respiration Machine Cheered in Brooklyn. Women in the Audience, But Most of Those Present Were Physicians — The Dog, Gathered in from the Street, Wagged Its Tail.
External links
- Mechanical Ventilation at eMedicine, article on mechanical ventilation along with technical information.
- International Ventilator Users Network (IVUN), Resource of information for users of home mechanical ventilation.
- Mechanical Ventilation, (detailed, illustrated slideshow presentation), by Amirali Nader, MD FCCP, Critical Care Medicine, Suburban Hospital, Johns Hopkins Medicine.