Indium(III) bromide
Indium(III) bromide, (indium tribromide), InBr3, is a chemical compound of indium and bromine. It is a Lewis acid and has been used in organic synthesis.[2]
 | |
| Names | |
|---|---|
| IUPAC name
Indium(III) bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.343 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| InBr3 | |
| Molar mass | 354.530 g/mol |
| Appearance | hygroscopic yellow-white monoclinic crystals |
| Density | 4.74 g/cm3 |
| Melting point | 420 °C (788 °F; 693 K) |
| 414 g/100 mL at 20 °C | |
| −107.0·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-428.9 kJ·mol−1 |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H314, H315, H319, H335 | |
| P260, P261, P264, P271, P280, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
| Related compounds | |
Other cations |
indium(III) fluoride indium(III) chloride indium(III) iodide |
Related compounds |
Indium(I) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
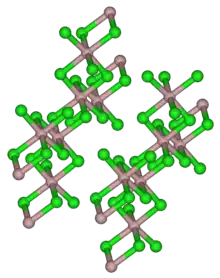
Structure
It has the same crystal structure as aluminium trichloride, with 6 coordinate indium atoms.[3] When molten it is dimeric, In2Br6, and it is predominantly dimeric in the gas phase. The dimer has bridging bromine atoms with a structure similar to dimeric aluminium trichloride Al2Cl6.[3]
Preparation and reactions
It is formed by the reaction of indium and bromine.[4] InBr3 forms complexes with ligands, L, InBr3L, InBr3L2, InBr3L3.[3]
Reaction with indium metal forms lower valent indium bromides, InBr2, In4Br7, In2Br3, In5Br7, In7Br9, indium(I) bromide.[5][6][7][8] In refluxing xylene solution InBr3 and In metal react to form InBr2.[9]
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–61, ISBN 0-8493-0594-2
- Thirupathi, Ponnaboina; Kim, Sung Soo (2009). "InBr3: A Versatile Catalyst for the Different Types of Friedel−Crafts Reactions". The Journal of Organic Chemistry. 74 (20): 7755–7761. doi:10.1021/jo9014613. ISSN 0022-3263. PMID 19813765.
- "Indium: Inorganic chemistry", D.G Tuck, Encyclopedia of Inorganic Chemistry Editor R Bruce King (1994) John Wiley and Sons ISBN 0-471-93620-0
- Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- Staffel, Thomas; Meyer, Gerd (1987). "The mono-, sesqui-, and dibromides of indium: InBr, In2Br3, and InBr2". Zeitschrift für anorganische und allgemeine Chemie. 552 (9): 113–122. doi:10.1002/zaac.19875520913. ISSN 0044-2313.
- Ruck, Michael; Bärnighausen, Hartmut (1999). "Zur Polymorphie von In5Br7". Zeitschrift für anorganische und allgemeine Chemie. 625 (4): 577–585. doi:10.1002/(SICI)1521-3749(199904)625:4<577::AID-ZAAC577>3.0.CO;2-B. ISSN 0044-2313.
- Dronskowski, R. (1995). "The crystal structure of In7Br9". Zeitschrift für Kristallographie. 210 (12): 920–923. doi:10.1524/zkri.1995.210.12.920. ISSN 0044-2968.
- Stephenson, NC; Mellor, DP (1950). "The Crystal Structure of Indium Monobromide". Australian Journal of Chemistry. 3 (4): 581. doi:10.1071/CH9500581. ISSN 0004-9425.
- Freeland, B. H.; Tuck, D. G. (1976). "Facile synthesis of the lower halides of indium". Inorganic Chemistry. 15 (2): 475–476. doi:10.1021/ic50156a050. ISSN 0020-1669.