Strontium bromide
Strontium bromide is a chemical compound with a formula SrBr2. At room temperature it is a white, odourless, crystalline powder. Strontium bromide imparts a bright red colour in a flame test, showing the presence of strontium ions. It is used in flares and also has some pharmaceutical uses.
 | |
| Names | |
|---|---|
| IUPAC name
Strontium bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.868 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| SrBr2 | |
| Molar mass | 247.428 g/mol (anhydrous) 355.53 g/mol (hexahydrate) |
| Appearance | white crystalline powder |
| Density | 4.216 g/cm3 (anhydrous) 2.386 g/cm3 (hexahydrate) |
| Melting point | 643 °C (1,189 °F; 916 K) |
| Boiling point | 2,146 °C (3,895 °F; 2,419 K) |
| 107 g/100 mL | |
| Solubility | soluble in alcohol insoluble in ether |
| −86.6·10−6 cm3/mol | |
| Structure | |
| tetragonal[1] | |
| Hazards | |
| Main hazards | Corrosive |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
strontium fluoride strontium chloride strontium iodide |
Other cations |
Beryllium bromide Magnesium bromide Calcium bromide Barium bromide Radium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
SrBr2 can be prepared from strontium hydroxide and hydrobromic acid.
Alternatively strontium carbonate can also be used as strontium source.
These reactions give hexahydrate of SrBr2, which decomposes to dihydrate at 89 °C. At 180 °C anhydrous SrBr2 is obtained.[2]
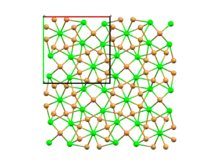
Structure
At room temperature, strontium bromide adopts a crystal structure with a tetragonal unit cell and space group P4/n. This structure is referred to as α-SrBr2 and is isostructural with EuBr2 and USe2. Around 920 K (650 °C), α-SrBr2 undergoes a first-order solid-solid phase transition to a much less ordered phase, β-SrBr2, which adopts the cubic fluorite structure. The beta phase of strontium bromide has a much higher ionic conductivity of about 1 S cm−1, comparable to that of molten SrBr2, due to extensive disorder in the bromide sublattice.[3] Strontium bromide melts at 930 K (657 °C).
 Space-filling model of the packing of Sr2+ and Br− ions in α-SrBr2
Space-filling model of the packing of Sr2+ and Br− ions in α-SrBr2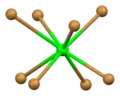 Distorted square antiprismatic coordination geometry of crystallographically independent strontium atom number 1
Distorted square antiprismatic coordination geometry of crystallographically independent strontium atom number 1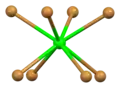 Square antiprismatic coordination geometry of strontium number 2
Square antiprismatic coordination geometry of strontium number 2 Flattened tetrahedral coordination geometry of bromine number 1
Flattened tetrahedral coordination geometry of bromine number 1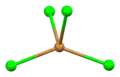 Distorted tetrahedral coordination geometry of bromine number 2
Distorted tetrahedral coordination geometry of bromine number 2 Tetrahedral coordination geometry of bromine number 3
Tetrahedral coordination geometry of bromine number 3 Tetrahedral coordination geometry of bromine number 4
Tetrahedral coordination geometry of bromine number 4
See also
References
- R. L. Sass; et al. (1963). "The crystal structure of strontium bromide". J. Phys. Chem. 67 (12): 2862. doi:10.1021/j100806a516.
- Dale L. Perry, Sidney L. Phillips: Handbook of Inorganic Compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, (Strontium bromide, p. 387, at Google Books).
- Hull, Stephen; Norberg, Stefan T.; Ahmed, Istaq; Eriksson, Sten G.; Mohn, Chris E. (2011). "High temperature crystal structures and superionic properties of SrCl2, SrBr2, BaCl2 and BaBr2". J. Solid State Chem. 184 (11): 2925–2935. doi:10.1016/j.jssc.2011.09.004.
