Trimethylaminuria
Trimethylaminuria (TMAU), also known as fish odor syndrome or fish malodor syndrome,[1] is a rare metabolic disorder that causes a defect in the normal production of an enzyme named flavin-containing monooxygenase 3 (FMO3).[2][3] When FMO3 is not working correctly or if not enough enzyme is produced, the body loses the ability to properly convert trimethylamine (TMA) from precursor compounds in food digestion into trimethylamine oxide (TMAO), through a process called N-oxidation. Trimethylamine then builds up and is released in the person's sweat, urine, and breath, giving off a strong fishy odor or strong body odor. A variant of TMAU (secondary trimethylaminuria or TMAU2) exists where there is no genetic cause, yet excessive TMA is secreted, possibly due to intestinal dysbiosis, altered metabolism, or hormonal causes.[4]
| Trimethylaminuria | |
|---|---|
| Other names | Primary trimethylaminuria |
 | |
| Trimethylamine | |
| Specialty | Endocrinology |
Symptoms
Trimethylamine builds up in the bodies of patients with trimethylaminuria. The trimethylamine is released in the person's sweat, urine, reproductive fluids, and breath, giving off a strong fishy or body odor. Some people with trimethylaminuria have a strong odor all the time, but most have a moderate smell that varies in intensity over time. Individuals with this condition do not have any physical symptoms, and they typically appear healthy.[5]
The condition seems to be more common in women than men, for unknown reasons. Scientists suspect that such female sex hormones as progesterone and estrogen aggravate the condition. According to several reports, the condition worsens around puberty. In women, symptoms may worsen just before and during menstrual periods, after taking oral contraceptives, and around menopause.[5]
The odor seems to vary depending on many known factors, including diet, hormonal changes, stress level, amount of sweat, other odors in the space, and the observer's sense of smell.
Genetics
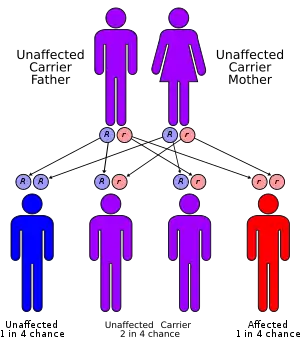
Most cases of trimethylaminuria appear to be inherited in an autosomal recessive pattern, which means two copies of the gene in each cell are altered. The parents of an individual with an autosomal recessive disorder are both carriers of one copy of the altered gene. Carriers may have mild symptoms of trimethylaminuria or experience temporary episodes of fish-like body odor.
Mutations in the FMO3 gene, which is found on the long arm of chromosome 1, cause trimethylaminuria. The FMO3 gene makes an enzyme that breaks down nitrogen-containing compounds from the diet, including trimethylamine. These compounds are produced by bacteria in the intestine as they digest proteins from eggs, meat, soy, and other foods. Normally, the FMO3 enzyme converts fishy-smelling trimethylamine into trimethylamine N-oxide which has no odor. If the enzyme is missing or its activity is reduced because of a mutation in the FMO3 gene, trimethylamine is not broken down and instead builds up in the body. As the compound is released in a person's sweat, urine, and breath, it causes the strong odor characteristic of trimethylaminuria. Researchers believe that stress and diet also play a role in triggering symptoms.
There are more than 40 known mutations associated with TMAU.[6][7] Loss-of-function mutations, nonsense mutations, and missense mutations are three of the most common. Nonsense and missense mutations cause the most severe phenotypes.
Although FMO3 mutations account for most known cases of trimethylaminuria, some cases are caused by other factors. A fish-like body odor could result from an excess of certain proteins in the diet or from an increase in bacteria in the digestive system. A few cases of the disorder have been identified in adults with liver damage caused by hepatitis.
In 2007 the evolution of the FMO3 gene was studied, including the evolution of some mutations associated with TMAU.[8]
Diagnosis
Measurement of urine for the ratio of trimethylamine to trimethylamine oxide is the standard screening test. A blood test is available to provide genetic analysis. The prominent enzyme responsible for TMA N-oxygenation is coded by the FMO3 gene.
False positives can occur in the following conditions, where elevated TMA can be present in the urine without any underlying TMAU:
- Urinary tract infection[9]
- Bacterial vaginosis[9]
- Cervical cancer[9]
- Advanced liver or kidney disease[9]
A similar foul-smelling odor of the urine has also been associated with colonization of the urinary tract with a bacterium called Aerococcus urinae, especially in children.[10]
Treatment
There is no known cure or treatment for the disorder.
The metabolic and clinical manifestations of TMAU are generally regarded as benign, as there is no associated organ dysfunction. This designation, and the fact that the condition is often unrecognised by doctors, misdiagnosed and can have important ramifications including missed or delayed diagnosis.[11]
Affected individuals experience shame and embarrassment, fail to maintain relationships, avoid contact with people who comment on their condition, and are obsessive about masking the odour with hygiene products and even smoking. The malodorous aspect can have serious and destructive effects on schooling, personal life, career and relationships, resulting in social isolation, low self-esteem, depression, paranoid behaviour, and suicide. Delayed diagnosis, body odour and the lack of cure may lead to psychosocial issues. When the condition is suspected or known to occur in a family, genetic testing can be helpful in identifying the specific individuals who have or carry the disorder.[11]
Ways of reducing the fishy odor may include:
- Avoiding foods such as egg yolks, legumes, red meats, fish, beans and other foods that contain choline, carnitine, nitrogen, sulfur and lecithin
- Taking low doses of antibiotics such as neomycin and metronidazole[12] in order to reduce the amount of bacteria in the gut
- Using slightly acidic detergent with a pH between 5.5 and 6.5
Additionally, at least one study[13] has suggested that daily intake of the supplements activated charcoal and copper chlorophyllin may improve the quality of life of individuals afflicted with TMAU by helping their bodies to oxidize and convert TMA to the odorless N-oxide (TMAO) metabolite. Study participants experienced subjective reduction in odor as well as objective reduction in TMA and increase in TMAO concentration measured in their urine. The study found that:
- 85% of test participants experienced complete loss of detectable "fishy" odor
- 10% experienced some reduction in detectable odor
- 5% did not experience any detectable odor reduction
History
The first clinical case of TMAU was described in 1970.[14]
References
- Mitchell SC, Smith RL (2001). "Trimethylaminuria: the fish malodor syndrome". Drug Metab Dispos. 29 (4 Pt 2): 517–21. PMID 11259343.
- Treacy EP, et al. (1998). "Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication". Human Molecular Genetics. 7 (5): 839–45. doi:10.1093/hmg/7.5.839. PMID 9536088.
- Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E (1999). "Mild trimethylaminuria caused by common variants in FMO3 gene". Lancet. 354 (9181): 834–5. doi:10.1016/S0140-6736(99)80019-1. PMID 10485731. S2CID 9555588.
- Mackay RJ, McEntyre CJ, Henderson C, Lever M, George PM (2011). "Trimethylaminuria: Causes and Diagnosis of a Socially Distressing Condition". Clin Biochem Rev. 32 (1): 33–43. PMC 3052392. PMID 21451776.CS1 maint: multiple names: authors list (link)
- "Learning About Trimethylaminuria". Retrieved 25 April 2016.
- Hernandez D, Addou S, Lee D, Orengo C, Shephard EA, Phillips IR (2003). "Trimethylaminuria and a human FMO3 mutation database". Hum Mutat. 22 (3): 209–13. doi:10.1002/humu.10252. PMID 12938085.
- Furnes B, Feng J, Sommer SS, Schlenk D (2003). "Identification of novel variants of the flavin-containing monooxygenase gene family in African Americans". Drug Metab Dispos. 31 (2): 187–93. doi:10.1124/dmd.31.2.187. PMID 12527699.
- Allerston CK, Shimizu M, Fujieda M, Shephard EA, Yamazaki H, Phillips IR (2007). "Molecular evolution and balancing selection in the flavin-containing monooxygenase 3 gene (FMO3)". Pharmacogenet Genomics. 17 (10): 827–39. doi:10.1097/FPC.0b013e328256b198. PMID 17885620. S2CID 6712355.
- Shephard, Elizabeth A; Treacy, Eileen P; Phillips, Ian R (30 November 2011). "Clinical utility gene card for: Trimethylaminuria". European Journal of Human Genetics. 20 (3): 4–5. doi:10.1038/ejhg.2011.214. PMC 3283181. PMID 22126753.
- Lenherr N, Berndt A, Ritz N, Rudin C. Aerococcus urinae: a possible reason for malodorous urine in otherwise healthy children. Eur J Pediatr. 2014;173:1115-7; Gibb AP, Sivaraman B. A second case of foul smelling urine in a boy caused by Aerococcus urinae. Pediatr Infect Dis J. 2013;32:1300-1.
- Treacy E; Johnson D. Pitt JJ; Danks DM (1995). "Trimethylaminuria, fish odour syndrome: A new method of detection and response to treatment with metronidazole". J Inherit Metab Dis. 18 (3): 306–12. doi:10.1007/bf00710420. PMID 7474897. S2CID 42397848.
- Yamazaki H, Fujieda M, Togashi M, et al. (2004). "Effects of the dietary supplements, activated charcoal and copper chlorophyllin, on urinary excretion of trimethylamine in Japanese trimethylaminuria patients". Life Sci. 74 (22): 2739–47. doi:10.1016/j.lfs.2003.10.022. PMID 15043988.
- Humbert JA, Hammond KB, Hathaway WE (1970). "Trimethylaminuria: the fish-odour syndrome". Lancet. 2 (7676): 770–1. doi:10.1016/S0140-6736(70)90241-2. PMID 4195988.
External links
| Classification | |
|---|---|
| External resources |
This article incorporates public domain text from The U.S. National Library of Medicine and The National Human Genome Research Institute