Lecithin
Lecithin (/ˈlɛsɪθɪn, ˈlɛsəθ-/, from the Greek lekithos "yolk") is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues which are amphiphilic – they attract both water and fatty substances (and so are both hydrophilic and lipophilic), and are used for smoothing food textures, emulsifying, homogenizing liquid mixtures, and repelling sticking materials.[1][2]
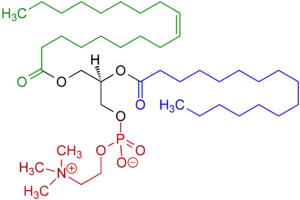
Lecithins are mixtures of glycerophospholipids including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and phosphatidic acid.[3]
Lecithin was first isolated in 1845 by the French chemist and pharmacist Théodore Gobley.[4] In 1850, he named the phosphatidylcholine lécithine.[5] Gobley originally isolated lecithin from egg yolk—λέκιθος lekithos is "egg yolk" in Ancient Greek—and established the complete chemical formula of phosphatidylcholine in 1874;[6] in between, he had demonstrated the presence of lecithin in a variety of biological matters, including venous blood, in human lungs, bile, human brain tissue, fish eggs, fish roe, and chicken and sheep brain.
Lecithin can easily be extracted chemically using solvents such as hexane, ethanol, acetone, petroleum ether or benzene; or extraction can be done mechanically. It is usually available from sources such as egg yolk,[7] marine sources, soybeans,[7] milk, rapeseed, cottonseed, and sunflower oil. It has low solubility in water, but is an excellent emulsifier. In aqueous solution, its phospholipids can form either liposomes, bilayer sheets, micelles, or lamellar structures, depending on hydration and temperature. This results in a type of surfactant that usually is classified as amphipathic. Lecithin is sold as a food additive and dietary supplement. In cooking, it is sometimes used as an emulsifier and to prevent sticking, for example in non-stick cooking spray.
Production
Commercial lecithin, as used by food manufacturers, is a mixture of phospholipids in oil. The lecithin can be obtained by water degumming the extracted oil of seeds. It is a mixture of various phospholipids, and the composition depends on the origin of the lecithin. A major source of lecithin is soybean oil. Because of the EU requirement to declare additions of allergens in foods, in addition to regulations regarding genetically modified crops, a gradual shift to other sources of lecithin (such as sunflower lecithin) is taking place. The main phospholipids in lecithin from soy and sunflower are phosphatidyl choline, phosphatidyl inositol, phosphatidyl ethanolamine, phosphatidylserine, and phosphatidic acid. They often are abbreviated to PC, PI, PE, PS and PA, respectively. Purified phospholipids are produced by companies commercially.
Hydrolysed lecithin
To modify the performance of lecithin to make it suitable for the product to which it is added, it may be hydrolysed enzymatically. In hydrolysed lecithins, a portion of the phospholipids have one fatty acid removed by phospholipase. Such phospholipids are called lysophospholipids. The most commonly used phospholipase is phospholipase A2, which removes the fatty acid at the C2 position of glycerol. Lecithins may also be modified by a process called fractionation. During this process, lecithin is mixed with an alcohol, usually ethanol. Some phospholipids, such as phosphatidylcholine, have good solubility in ethanol, whereas most other phospholipids do not dissolve well in ethanol. The ethanol is separated from the lecithin sludge, after which the ethanol is removed by evaporation to obtain a phosphatidylcholine-enriched lecithin fraction.
Genetically modified crops as a source of lecithin
As described above, lecithin is highly processed. Therefore, genetically modified (GM) protein or DNA from the original GM crop from which it is derived often is undetectable – in other words, it is not substantially different from lecithin derived from non-GM crops.[8] Nonetheless, consumer concerns about genetically modified food have extended to highly purified derivatives from GM food, such as lecithin. This concern led to policy and regulatory changes in the EU in 2000, when Commission Regulation (EC) 50/2000 was passed[10] which required labelling of food containing additives derived from GMOs, including lecithin. Because it is nearly impossible to detect the origin of derivatives such as lecithin, the European regulations require those who wish to sell lecithin in Europe to use a meticulous, but essential system of identity preservation (IP).[8][11]
Properties and applications

Lecithins have emulsification and lubricant properties, and are a surfactant. They can be completely metabolized (see inositol) by humans, so are well tolerated by humans and nontoxic when ingested; some other emulsifiers can only be excreted via the kidneys.[12]
The major components of commercial soybean-derived lecithin are:[13]
- 33–35% Soybean oil
- 20–21% Phosphatidylinositols
- 19–21% Phosphatidylcholine
- 8–20% Phosphatidylethanolamine
- 5–11% Other phosphatides
- 5% Free carbohydrates
- 2–5% Sterols
- 1% Moisture
Lecithin is used for applications in human food, animal feed, pharmaceuticals, paints, and other industrial applications.
Applications include:
- In the pharmaceutical industry, it acts as a wetting agent, stabilizing agent and a choline enrichment carrier, helps in emulsification and encapsulation, and is a good dispersing agent. It can be used in manufacture of intravenous fat infusions and for therapeutic use.
- In animal feed, it enriches fat and protein and improves pelletization.
- In the paint industry, it forms protective coatings for surfaces with painting and printing ink, has antioxidant properties, helps as a rust inhibitor, is a colour-intensifying agent, catalyst, conditioning aid modifier, and dispersing aid; it is a good stabilizing and suspending agent, emulsifier, and wetting agent, helps in maintaining uniform mixture of several pigments, helps in grinding of metal oxide pigments, is a spreading and mixing aid, prevents hard settling of pigments, eliminates foam in water-based paints, and helps in fast dispersion of latex-based paints.
- Lecithin also may be used as a release agent for plastics, an antisludge additive in motor lubricants, an antigumming agent in gasoline, and an emulsifier, spreading agent, and antioxidant in textile, rubber, and other industries.
Food additive
The nontoxicity of lecithin leads to its use with food, as an additive or in food preparation. It is used commercially in foods requiring a natural emulsifier or lubricant.
In confectionery, it reduces viscosity, replaces more expensive ingredients, controls sugar crystallization and the flow properties of chocolate, helps in the homogeneous mixing of ingredients, improves shelf life for some products, and can be used as a coating. In emulsions and fat spreads, such as margarines with a high fat content of more than 75%, it stabilizes emulsions, reduces spattering (splashing and scattering of oil droplets) during frying, improves texture of spreads and flavor release.[14] In doughs and baking, it reduces fat and egg requirements, helps even out distribution of ingredients in dough, stabilizes fermentation, increases volume, protects yeast cells in dough when frozen, and acts as a releasing agent to prevent sticking and simplify cleaning. It improves wetting properties of hydrophilic powders (such as low-fat proteins) and lipophilic powders (such as cocoa powder), controls dust, and helps complete dispersion in water.[15] Lecithin keeps cocoa and cocoa butter in a candy bar from separating. It can be used as a component of cooking sprays to prevent sticking and as a releasing agent.
Lecithin is approved by the United States Food and Drug Administration for human consumption with the status "generally recognized as safe". Lecithin is admitted by the EU as a food additive, designated as E322.[16]
Dietary supplement
Because it contains phosphatidylcholines, lecithin is a source of choline, an essential nutrient.[17] Clinical studies have shown benefit in acne, in improving liver function, and in lowering cholesterol, but older clinical studies in dementia and dyskinesias had found no benefit.[18]
An earlier study using a small sample (20 men divided in 3 groups) did not detect statistically significant short term (2–4 weeks) effects on cholesterol in hyperlipidemic men.[19]
La Leche League recommends its use to prevent blocked or plugged milk ducts which can lead to mastitis in breastfeeding women.[20]
Egg-derived lecithin is not usually a concern for those allergic to eggs since commercially available egg lecithin is highly purified and devoid of allergy-causing egg proteins.[21] Similarly, soy lecithin does not contain enough allergenic proteins for most people allergic to soy, although the US FDA only exempts a few soy lecithin products from its mandatory allergenic source labeling requirements.[22]
Religious restrictions
Soy-derived lecithin is considered by some to be kitniyot and prohibited on Passover for Ashkenazi Jews when many grain-based foods are forbidden, but not at other times. This does not necessarily affect Sephardi Jews, who do not have the same restrictions on rice and kitniyot during Passover.[23]
Muslims are not forbidden to eat lecithin per se; however, since it may be derived from animal as well as plant sources, care must be taken to ensure this source is halal. Lecithin derived from plants and egg yolks is permissible, as is that derived from animals slaughtered according to the rules of dhabihah.[24]
Research
Research suggests soy-derived lecithin has significant effects on lowering serum cholesterol and triglycerides, while increasing HDL ("good cholesterol") levels in the blood of rats.[25][26] However, a growing body of evidence indicates the phosphatidylcholine in lecithin is converted by gut bacteria into trimethylamine N-oxide (TMAO), which is absorbed by the gut and may with time contribute to atherosclerosis and heart attacks.[27][28][29] There is also some preliminary evidence suggesting that excessive consumption of lecithin, either via foodstuffs or supplements, may promote depression in sensitive individuals.[30]
See also
References
- "Lecithin". Merriam Webster Dictionary Online.
- Szuha, Bernard F. (1989). "Chapter 7". Lecithins: Sources, Manufacture & Uses. The American Oil Chemist's Society. p. 109. ISBN 0-935315-27-6.
- Smith, Jim; Hong-Shum, Lily, eds. (2011). Food Additives Data Book (2nd ed.). Chichester, West Sussex: Wiley-Blackwell. p. 334. ISBN 9781444397734.
Complex mixture of phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidic acid, glycolipids, etc.
- Gobley, Théodore (1846). "Recherches chimiques sur le jaune d'œuf" [Chemical researches on egg yolk]. Journal de Pharmacie et de Chemie. 3rd series (in French). 9: 81–91.
- Gobley, Théodore (1850). "Recherches chemiques sur les œufs de carpe" [Chemical researches on carp eggs]. Journal de Pharmacie et de Chemie. 3rd series (in French). 17: 401–430.
Je propose de donner au premier le nom de Lécithine (de λεκιθος, jaune d'œuf), parce qu'on le rencontre en grande quantité dans le jaune d'œuf … (I propose to give to the former the name of lecithin (from λεκιθος, egg yolk), because it is encountered in great quantity in egg yolk … )
- Gobley, Théodore (1874). "Sur la lécithine et la cérébrine" [On lecithin and cerebrin]. Journal de Pharmacie et de Chimie. 4th series (in French). 19: 346–353.
- "Lecithin: Uses, Side Effects, Interactions, Dosage, and Warning". WebMD. 2019-01-30. Retrieved 2019-06-18.
- Marx, Gertruida M. (December 2010). Monitoring of genetically modified food products in South Africa (PDF) (PhD). University of the Free State. Archived from the original (PDF) on 2015-01-09.
- "Regulation (EC) 50/2000". europa.eu.
- Davison, John; Bertheau, Yves (2007). "EU regulations on the traceability and detection of GMOs: difficulties in interpretation, implementation, and compliance". CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition, and Natural Resources. 2 (77): 77. doi:10.1079/PAVSNNR20072077.
- "What are the Uses of Lecithin in Food?".
- Scholfield, C. R. (October 1981). "Composition of Soybean Lecithin". Journal of the American Oil Chemists' Society. 58 (10): 889–892. doi:10.1007/bf02659652. S2CID 9876375. Retrieved 2014-08-21 – via USDA.
- Gunstone, Frank D.; Harwood, John L.; Dijkstra, Albert J., eds. (2007). "Food Uses of Oils and Fats". The Lipid Handbook. CRC Press. p. 340. ISBN 978-0-8493-9688-5.
- Riehm, David A.; Rokke, David J.; Paul, Prakash G.; Lee, Han Seung; Vizanko, Brent S.; McCormick, Alon V. (2017-02-01). "Dispersion of oil into water using lecithin-Tween 80 blends: The role of spontaneous emulsification". Journal of Colloid and Interface Science. 487: 52–59. Bibcode:2017JCIS..487...52R. doi:10.1016/j.jcis.2016.10.010. ISSN 0021-9797. PMID 27744169.
- "Current EU approved additives and their E Numbers". UK Food Standards Agency. Retrieved 26 November 2010.
- Zeisel, S. H.; da Costa, K. A. (November 2009). "Choline: an essential nutrient for public health". Nutrition Reviews. 67 (11): 615–623. doi:10.1111/j.1753-4887.2009.00246.x. PMC 2782876. PMID 19906248.
- Higgins, J. P.; Flicker, L. (2003). "Lecithin for dementia and cognitive impairment". Cochrane Database of Systematic Reviews. 3 (3): CD001015. doi:10.1002/14651858.CD001015. PMID 12917896.
- Oosthuizen, W.; Vorster, H. H.; Vermaak, W. J.; et al. (1998). "Lecithin has no effect on serum lipoprotein, plasma fibrinogen and macro molecular protein complex levels in hyperlipidaemic men in a double-blind controlled study". European Journal of Clinical Nutrition. 52 (6): 419–424. doi:10.1038/sj.ejcn.1600580. PMID 9683394.
- Wiessinger, Diane; West, Diana; Pitman, Teresa (2010). "Dealing with Plugs and Blebs" (PDF). The Womanly Art of Breastfeeding. La Leche League. ISBN 978-0345518446. Archived from the original (PDF) on 2010-12-30.
- Discussion Forum: American Academy of Allergy, Asthma, and Immunology
- "Soy lecithin". Food Allergy Research and Resource Program. University of Nebraska–Lincoln. Retrieved 14 December 2018.
- (Reb Yehonatan Levy, Shomer Kashrut Mashgiach - based upon halachic rulings of CRC - Chicago Rabbinic Council, and from shiurim/lessons by Rabbi D. Raccah on "Pesach Preparations" following commentary from former Rishon-LeTzion Rav Ovadia Yosef). OK Kosher Certification, Keeping Kosher for Pesach. Retrieved on September 10, 2008.
- Islamic Food and Nutrition Council of America FAQ, IFANCA: Consumer FAQ. Archived 2011-11-23 at the Wayback Machine Retrieved on July 7, 2010. The practice of consuming Halal products is not widespread among Muslims, the practice is common with Muslims who follow Sharia laws.
- Iwata T, Kimura Y, Tsutsumi K, Furukawa Y, Kimura S (February 1993). "The effect of various phospholipids on plasma lipoproteins and liver lipids in hypercholesterolemic rats". J. Nutr. Sci. Vitaminol. 39 (1): 63–71. doi:10.3177/jnsv.39.63. PMID 8509902.
- Jimenez MA, Scarino ML, Vignolini F, Mengheri E (July 1990). "Evidence that polyunsaturated lecithin induces a reduction in plasma cholesterol level and favorable changes in lipoprotein composition in hypercholesterolemic rats". J. Nutr. 120 (7): 659–67. doi:10.1093/jn/120.7.659. PMID 2366101.
- Wendy R Russell WR et al. (2013) Colonic bacterial metabolites and human health (Review). Current Opinion in Microbiology 16(3):246–254
- Tang, WH; Wang Z; Levison BS; Koeth RA; Britt EB; Fu X; Wu Y; Hazen SL (Apr 25, 2013). "Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk". N Engl J Med. 368 (17): 1575–84. doi:10.1056/NEJMoa1109400. PMC 3701945. PMID 23614584.
- Mendelsohn, AR; Larrick JW (Jun 2013). "Dietary modification of the microbiome affects risk for cardiovascular disease". Rejuvenation Res. 16 (3): 241–4. doi:10.1089/rej.2013.1447. PMID 23656565.
- Tsoukalas I (2019). "Too much of a good thing? Lecithin and mental health". World Nutrition. 10 (1): 54–62. doi:10.26596/wn.201910154-62.pdf.
External links
- Introduction to Lecithin (University of Erlangen)
- FDA Industry guideline for soy lecithin labeling
- European Lecithin Manufacturers Association official website