11β-Hydroxytestosterone
11β-Hydroxytestosterone is an endogenous steroid, a metabolite of testosterone.[1][2][3]
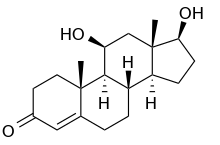 | |
| Names | |
|---|---|
| IUPAC name
11β,17β-dihydroxyandrost-4-ene-3-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.162.057 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H28O3 | |
| Molar mass | 304.430 g·mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H351, H360 | |
| P201, P202, P281, P308+313, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Storbeck KH, Mostaghel EA (2019). "Canonical and Noncanonical Androgen Metabolism and Activity". Advances in Experimental Medicine and Biology. 1210: 239–277. doi:10.1007/978-3-030-32656-2_11. ISBN 978-3-030-32655-5. PMID 31900912.
CYP11B1 and 2 have also been shown to 11β-hydroxylate T, yielding 11β-hydroxytestosterone (11OHT), though the levels produced by the adrenal are low due to the limited availability of adrenal derived T
- Stárka L, Dušková M, Vítků J (September 2020). "11-Keto-testosterone and other androgens of adrenal origin". Physiological Research. 69 (Suppl 2): S187–S192. doi:10.33549/physiolres.934516. PMID 33094617.
- Van Rooyen, D.; Gent, R.; Barnard, L.; Swart, A. C. (2018). "The in vitro metabolism of 11β-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology. 178: 203–212. doi:10.1016/j.jsbmb.2017.12.014. PMID 29277707. S2CID 3700135.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
