18-Hydroxycortisol
18-Hydroxycortisol is an endogenous steroid.[1][2][3][4]
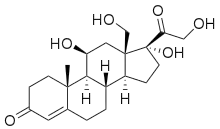 | |
| Names | |
|---|---|
| IUPAC name
(11β,17α)-11,17,18,21-Tetrahydroxypregn-4-ene-3,20-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H30O6 | |
| Molar mass | 378.465 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Function
18-hydroxycortisol has been proposed as a biomarker for certain diseases. In humans, 18-hydroxycortisol has no biological activity on glucocorticoid or mineralocorticoid receptors. In healthy subjects, the biosynthesis of 18-hydroxycortisol is low. The highest synthesis of 18-hydroxycortisol was found in certain cases of hypertension like in type 1 familial hyperaldosteronism (glucocorticoid-curable hyperaldosteronism) and type 3 familial hyperaldosteronism, where the adrenal glands are enlarged up to six times their normal size. Increased synthesis is also found in patients with aldosterone-producing adenomas. ACTH stimulation test increases urinary excretion of 18-hydroxycortisol, and dexamethasone inhibits the excretion.[1]
References
- Lenders J, Williams T, Reincke M, Gomez-Sanchez C (January 2018). "18-Oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids?". European Journal of Endocrinology. 178 (1): R1–R9. doi:10.1530/EJE-17-0563. PMC 5705277. PMID 28904009.
- Jin S, Wada N, Takahashi Y, Hui SP, Sakurai T, Fuda H, Takeda S, Fujikawa M, Yanagisawa K, Ikegawa S, Kurosawa T, Chiba H (September 2013). "Quantification of urinary 18-hydroxycortisol using LC-MS/MS". Annals of Clinical Biochemistry. 50 (Pt 5): 450–6. doi:10.1177/0004563213476272. PMID 23847032.
- Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, Veglio F (March 2012). "18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes". The Journal of Clinical Endocrinology and Metabolism. 97 (3): 881–9. doi:10.1210/jc.2011-2384. PMID 22238407.
- Chiba H (July 2010). "18-Hydroxycortisol, 18-oxocortisol, and 6beta-hydroxycortisol". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 68 Suppl 7: 339–43. PMID 20963880.