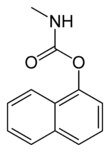
Carbaryl
Carbaryl (1-naphthyl methylcarbamate) is a chemical in the carbamate family used chiefly as an insecticide. It is a white crystalline solid commonly sold under the brand name Sevin, a trademark of the Bayer Company. Union Carbide discovered carbaryl and introduced it commercially in 1958. Bayer purchased Aventis CropScience in 2002, a company that included Union Carbide pesticide operations. It remains the third-most-used insecticide in the United States for home gardens, commercial agriculture, and forestry and rangeland protection. About 11 million kilograms were applied to U.S. farm crops in 1976.[3] As a veterinary drug, it is known as carbaril (INN).
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-naphthyl methylcarbamate | |||
| Preferred IUPAC name
naphthalen-1-yl methylcarbamate | |||
| Other names
Sevin (Generic trademark) α-Naphthyl N-methylcarbamate | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.505 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2757 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C12H11NO2 | |||
| Molar mass | 201.225 g·mol−1 | ||
| Appearance | Colorless crystalline solid | ||
| Density | 1.2 g/cm3 | ||
| Melting point | 142 °C (288 °F; 415 K) | ||
| Boiling point | decomposes | ||
| very low (0.01% at 20°C)[1] | |||
| Pharmacology | |||
| QP53AE01 (WHO) | |||
| Hazards | |||
| Safety data sheet | ICSC 0121 | ||
| GHS pictograms |     | ||
| GHS Signal word | Danger | ||
| H301, H302, H332, H351, H400, H410 | |||
| P201, P202, P261, P264, P270, P271, P273, P281, P301+310, P301+312, P304+312, P304+340, P308+313, P312, P321, P330, P391, P405, P501 | |||
| Flash point | 193-202 [1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
710 mg/kg (rabbit, oral) 250 mg/kg (guinea pig, oral) 850 mg/kg (rat, oral) 759 mg/kg (dog, oral) 500 mg/kg (rat, oral) 150 mg/kg (cat, oral) 128 mg/kg (mouse, oral) 230 mg/kg (rat, oral)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 5 mg/m3[1] | ||
REL (Recommended) |
TWA 5 mg/m3[1] | ||
IDLH (Immediate danger) |
100 mg/m3[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
.jpg.webp)
Production
Carbaryl is often inexpensively produced by direct reaction of methyl isocyanate with 1-naphthol.[4]
- C10H7OH + CH3NCO → C10H7OC(O)NHCH3
Alternatively, 1-naphthol can be treated with excess phosgene to produce 1-naphthylchloroformate, which is then converted to carbaryl by reaction with methylamine.[4] The former process was carried out in Bhopal. In comparison, the latter synthesis uses exactly the same reagents, but in a different sequence. This procedure avoids the potential hazards of methyl isocyanate.
Biochemistry
Carbamate insecticides are slowly reversible inhibitors of the enzyme acetylcholinesterase. They resemble acetylcholine, but the carbamoylated enzyme undergoes the final hydrolysis step very slowly (minutes) compared with the acetylated enzyme generated by acetylcholine (microseconds). They interfere with the cholinergic nervous system and cause death because the effects of the neurotransmitter acetylcholine cannot be terminated by carbamoylated acetylcholinesterase.
Applications
The development of the carbamate insecticides has been called a major breakthrough in pesticides. The carbamates do not have the persistence of chlorinated pesticides. Although toxic to insects, carbaryl is detoxified and eliminated rapidly in vertebrates. It is neither concentrated in fat nor secreted in milk, so is favored for food crops, at least in the US.[3] It is the active ingredient in Carylderm shampoo used to combat head lice until infestation is eliminated.
Ecology
Carbaryl kills both targeted (e.g., malaria-carrying mosquitos) and beneficial insects (e.g., honeybees), as well as crustaceans.[5]
Although approved for more than 100 crops in the US, carbaryl is illegal in the United Kingdom, Austria, Denmark, Sweden, Iran, Germany, and Angola.[6]
Safety
Carbaryl is a cholinesterase inhibitor and is toxic to humans. It is classified as a likely human carcinogen by the United States Environmental Protection Agency (EPA.)[7] The oral LD50 is 250 to 850 mg/kg for rats and 100 to 650 mg/kg for mice.
Carbaryl can be produced using methyl isocyanate (MIC) as an intermediary.[4] A leak of MIC used in the production of carbaryl caused the Bhopal disaster, the most lethal industrial accident in history.[8]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0100". National Institute for Occupational Safety and Health (NIOSH).
- "Carbaryl". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Robert L. Metcalf “Insect Control” in Ullmann’s Encyclopedia of Industrial Chemistry” Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a14_263
- Thomas A. Unger (1996). Pesticide Synthesis Handbook (Google Books excerpt). William Andrew. pp. 67–68. ISBN 0-8155-1401-8.
- Carbaryl Insecticide Hazard Data Archived May 11, 2010, at the Wayback Machine
- Interim Reregistration Eligibility Decision for Carbaryl Archived July 25, 2008, at the Wayback Machine, U.S. EPA, June 2003.
- Eckerman, Ingrid (2005). The Bhopal Saga—Causes and Consequences of the World's Largest Industrial Disaster. India: Universities Press. doi:10.13140/2.1.3457.5364. ISBN 81-7371-515-7.
External links
- Carbaryl Technical Fact Sheet - National Pesticide Information Center
- Carbaryl General Fact Sheet - National Pesticide Information Center
- Carbaryl Pesticide Information Profile - Extension Toxicology Network
- Cholinesterase Inhibition - Extension Toxicology Network
- EPA info
- EPA factsheet
- IPCS (WHO) Health and Safety Guide
- Environmental Health Criteria - WHO
- Exclusive Chemistry Ltd - routes of Sevin synthesis
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Carbaryl in the Pesticide Properties DataBase (PPDB)