Dissolved organic carbon
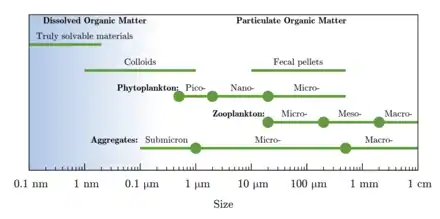
Dissolved organic carbon (DOC) is the fraction of organic carbon operationally defined as that which can pass through a filter with a pore size typically between 0.22 and 0.7 micrometers.[2] The fraction remaining on the filter is called particulate organic carbon (POC).[3]
| Part of a series on the |
| Carbon cycle |
|---|
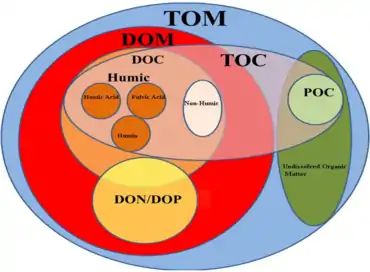 |
Dissolved organic matter (DOM) is a closely related term often used interchangeably with DOC. While DOC refers specifically to the mass of carbon in the dissolved organic material, DOM refers to the total mass of the dissolved organic matter. So DOM also includes the mass of other elements present in the organic material, such as nitrogen, oxygen and hydrogen. DOC is a component of DOM and there is typically about twice as much DOM as DOC.[4] Many statements that can be made about DOC apply equally to DOM, and vice versa.
DOC is abundant in marine and freshwater systems and is one of the greatest cycled reservoirs of organic matter on Earth, accounting for the same amount of carbon as in the atmosphere and up to 20% of all organic carbon.[5] In general, organic carbon compounds are the result of decomposition processes from dead organic matter including plants and animals. DOC can originate from within or outside any given body of water. DOC originating from within the body of water is known as autochthonous DOC and typically comes from aquatic plants or algae, while DOC originating outside the body of water is known as allochthonous DOC and typically comes from soils or terrestrial plants.[6] When water originates from land areas with a high proportion of organic soils, these components can drain into rivers and lakes as DOC.
The marine DOC pool is important for the functioning of marine ecosystems because they are at the interface between the chemical and the biological worlds. DOC fuels marine food webs, and is a major component of the Earth’s carbon cycling.[7]
Overview

DOC is a basic nutrient, supporting growth of microorganisms and plays an important role in the global carbon cycle through the microbial loop.[10] In some organisms (stages) that do not feed in the traditional sense, dissolved matter may be the only external food source.[11] Moreover, DOC is an indicator of organic loadings in streams, as well as supporting terrestrial processing (e.g., within soil, forests, and wetlands) of organic matter. Dissolved organic carbon has a high proportion of biodegradable dissolved organic carbon (BDOC) in first order streams compared to higher order streams. In the absence of extensive wetlands, bogs, or swamps, baseflow concentrations of DOC in undisturbed watersheds generally range from approximately 1 to 20 mg/L carbon.[12] Carbon concentrations considerably vary across ecosystems. For example, the Everglades may be near the top of the range and the middle of oceans may be near the bottom. Occasionally, high concentrations of organic carbon indicate anthropogenic influences, but most DOC originates naturally.[13]
The BDOC fraction consists of organic molecules that heterotrophic bacteria can use as a source of energy and carbon. [14] Some subset of DOC constitutes the precursors of disinfection byproducts for drinking water.[15] BDOC can contribute to undesirable biological regrowth within water distribution systems.[16]
The dissolved fraction of total organic carbon (TOC) is an operational classification. Many researchers use the term "dissolved" for compounds that pass through a 0.45 μm filter, but 0.22 μm filters have also been used to remove higher colloidal concentrations.
A practical definition of dissolved typically used in marine chemistry is all substances that pass through a GF/F filter, which has a nominal pore size of approximately 0.7 μm (Whatman glass microfiber filter, 0.6–0.8 μm particle retention[17]). The recommended procedure is the HTCO technique, which calls for filtration through pre-combusted glass fiber filters, typically the GF/F classification.[18]
Labile and recalcitrant
Dissolved organic matter can be classified as labile or as recalcitrant, depending on its reactivity. Recalcitrant DOC is also called refractory DOC, and these terms seem to be used interchangeably in the context of DOC. Depending on the origin and composition of DOC, its behavior and cycling are different; the labile fraction of DOC decomposes rapidly through microbially or photochemically mediated processes, whereas refractory DOC is resistant to degradation and can persist in the ocean for millennia. In the coastal ocean, organic matter from terrestrial plant litter or soils appears to be more refractory[19] and thus often behaves conservatively. In addition, refractory DOC is produced in the ocean by the bacterial transformation of labile DOC, which reshapes its composition.[20][21][22]
Due to the continuous production and degradation in natural systems, the DOC pool contains a spectrum of reactive compounds each with their own reactivity,[23] that have been divided into fractions from labile to recalcitrant, depending on the turnover times,[24] as shown in the following table...
| DOC fraction | acronym | turnover time | amount |
|---|---|---|---|
| labile | DOCL | hours to days | < 200 Tg C |
| semi-labile | DOCSL | weeks to months | ∼600 Tg C |
| semi-recalcitrant | DOCSR | decades | ∼1400 Tg C |
| recalcitrant | DOCR | thousands of years | ∼63000 Tg C |
| highly resistant | tens of thousands of years | ||
This wide range in turnover or degradation times has been linked with the chemical composition, structure and molecular size,[25][26] but degradation also depends on the environmental conditions (e.g., nutrients), prokaryote diversity, redox state, iron availability, mineral-particle associations, temperature, sun-light exposure, biological production of recalcitrant compounds, and the effect of priming or dilution of individual molecules.[25][27][28][29][30][31] For example, lignin can be degraded in aerobic soils but is relatively recalcitrant in anoxic marine sediments.[32] This example shows bioavailability varies as a function of the ecosystem’s properties. Accordingly, even normally ancient and recalcitrant compounds, such as petroleum, carboxyl-rich alicyclic molecules, can be degraded in the appropriate environmental setting.[33][34]
Terrestrial ecosystems
Soil
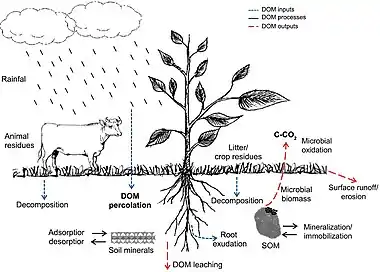
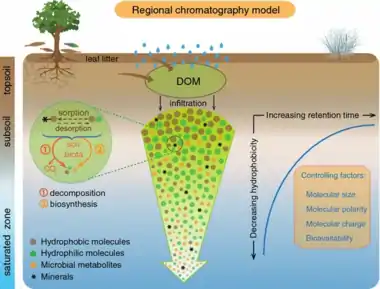
P = photosynthesis R = respiration
Dissolved organic matter (DOM) is one of the most active and mobile carbon pools and has an important role in global carbon cycling.[41] In addition, dissolved organic carbon (DOC) affects the soil negative electrical charges denitrification process, acid-base reactions in the soil solution, retention and translocation of nutrients (cations), and immobilization of heavy metals and xenobiotics.[42] Soil DOM can be derived from different sources (inputs), such as atmospheric carbon dissolved in rainfall, litter and crop residues, manure, root exudates, and decomposition of soil organic matter (SOM). In the soil, DOM availability depends on its interactions with mineral components (e.g., clays, Fe and Al oxides) modulated by adsorption and desorption processes.[43] It also depends on SOM fractions (e.g., stabilized organic molecules and microbial biomass) by mineralization and immobilization processes. In addition, the intensity of these interactions changes according to soil inherent properties,[44] land use, and crop management.[43][35]
During the decomposition of organic material, most carbon is lost as CO2 to the atmosphere by microbial oxidation. Soil type and landscape slope, leaching, and runoff are also important processes associated to DOM losses in the soil.[45] In well-drained soils, leached DOC can reach the water table and release nutrients and pollutants that can contaminate groundwater,[46][47] whereas runoff transports DOM and xenobiotics to other areas, rivers, and lakes.[35]
Groundwater
Precipitation and surface water leaches dissolved organic carbon (DOC) from vegetation and plant litter and percolates through the soil column to the saturated zone. The concentration, composition, and bioavailability of DOC are altered during transport through the soil column by various physicochemical and biological processes, including sorption, desorption, biodegradation and biosynthesis. Hydrophobic molecules are preferentially partitioned onto soil minerals and have a longer retention time in soils than hydrophilic molecules. The hydrophobicity and retention time of colloids and dissolved molecules in soils are controlled by their size, polarity, charge, and bioavailability. Bioavailable DOM is subjected to microbial decomposition, resulting in a reduction in size and molecular weight. Novel molecules are synthesized by soil microbes, and some of these metabolites enter the DOC reservoir in groundwater.[36]
Freshwater ecosystems
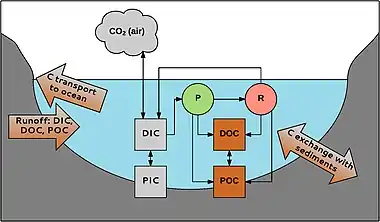
Aquatic carbon occurs in different forms. Firstly, a division is made between organic and inorganic carbon. Organic carbon is a mixture of organic compounds originating from detritus or primary producers. It can be divided into POC (particulate organic carbon; particles > 0.45 μm) and DOC (dissolved organic carbon; particles < 0.45 μm). DOC usually makes up 90% of the total amount of aquatic organic carbon. Its concentration ranges from 0.1 to >300 mg L-1.[48]
Likewise, inorganic carbon also consists of a particulate (PIC) and a dissolved phase (DIC). PIC mainly consists of carbonates (e.g., CaCO3), DIC consists of carbonate (CO32-), bicarbonate (HCO3-), CO2 and a negligibly small fraction of carbonic acid (H2CO3). The inorganic carbon compounds exist in equilibrium that depends on the pH of the water.[49] DIC concentrations in freshwater range from about zero in acidic waters to 60 mg C L-1 in areas with carbonate-rich sediments.[50]
POC can be degraded to form DOC; DOC can become POC by flocculation. Inorganic and organic carbon are linked through aquatic organisms. CO2 is used in photosynthesis (P) by for instance macrophytes, produced by respiration (R), and exchanged with the atmosphere. Organic carbon is produced by organisms and is released during and after their life; e.g., in rivers, 1–20% of the total amount of DOC is produced by macrophytes.[38] Carbon can enter the system from the catchment and is transported to the oceans by rivers and streams. There is also exchange with carbon in the sediments, e.g., burial of organic carbon, which is important for carbon sequestration in aquatic habitats.[51]
Aquatic systems are very important in global carbon sequestration; e.g., when different European ecosystems are compared, inland aquatic systems form the second largest carbon sink (19–41 Tg C y-1); only forests take up more carbon (125–223 Tg C y-1).[52][37]
Marine ecosystems
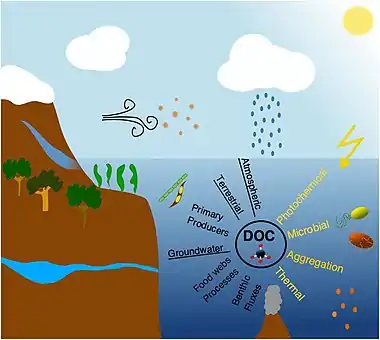
Sources
In marine systems DOC originates from either autochthonous or allochthonous sources. Autochthonous DOC is produced within the system, primarily by plankton organisms [53][54] and in coastal waters additionally by benthic microalgae, benthic fluxes, and macrophytes,[55] whereas allochthonous DOC is mainly of terrestrial origin supplemented by groundwater and atmospheric inputs.[56][57] In addition to soil derived humic substances, terrestrial DOC also includes material leached from plants exported during rain events, emissions of plant materials to the atmosphere and deposition in aquatic environments (e.g., volatile organic carbon and pollens), and also thousands of synthetic human-made organic chemicals that can be measured in the ocean at trace concentrations.[58][59][7]
Phytoplankton
Phytoplankton produces DOC by extracellular release commonly accounting between 5 and 30% of their total primary production,[60] although this varies from species to species.[61] Nonetheless, this release of extracellular DOC is enhanced under high light and low nutrient levels, and thus should increase relatively from eutrophic to oligotrophic areas, probably as a mechanism for dissipating cellular energy.[62] Phytoplankton can also produce DOC by autolysis during physiological stress situations e.g., nutrient limitation.[63] Other studies have demonstrated DOC production in association with meso- and macro-zooplankton feeding on phytoplankton and bacteria.[64][7]
Zooplankton
Zooplankton-mediated release of DOC occurs through sloppy feeding, excretion and defecation which can be important energy sources for microbes.[65][64] Such DOC production is largest during periods with high food concentration and dominance of large zooplankton species.[66][7]
Bacteria and viruses
Bacteria are often viewed as the main consumers of DOC, but they can also produce DOC during cell division and viral lysis.[67][68][69] The biochemical components of bacteria are largely the same as other organisms, but some compounds from the cell wall are unique and are used to trace bacterial derived DOC (e.g., peptidoglycan). These compounds are widely distributed in the ocean, suggesting that bacterial DOC production could be important in marine systems.[70] Viruses are the most abundant life forms in the oceans infecting all life forms including algae, bacteria and zooplankton.[71] After infection, the virus either enters a dormant (lysogenic) or productive (lytic) state.[72] The lytic cycle causes disruption of the cell(s) and release of DOC.[73][7]
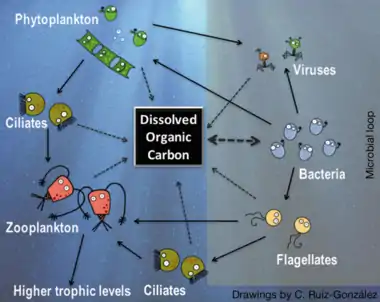
Right side: microbial loop, with bacteria using dissolved organic carbon to gain biomass, which then re-enters the classic carbon flow through protists.[74][75]
Macrophytes
Marine macrophytes (i.e., macroalgae and seagrass) are highly productive and extend over large areas in coastal waters but their production of DOC has not received much attention. Macrophytes release DOC during growth with a conservative estimate (excluding release from decaying tissues) suggesting that macroalgae release between 1-39% of their gross primary production,[77][78] while seagrasses release less than 5% as DOC of their gross primary production.[79] The released DOC has been shown to be rich in carbohydrates, with rates depending on temperature and light availability.[80][81] Globally the macrophyte communities have been suggested to produce ∼160 Tg C yr–1 of DOC, which is approximately half the annual global river DOC input (250 Tg C yr–1).[80][7]
Marine sediments
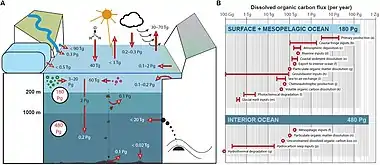
Marine sediments represent the main sites of OM degradation and burial in the ocean, hosting microbes in densities up to 1000 times higher than found in the water column.[83] The DOC concentrations in sediments are often an order of magnitude higher than in the overlying water column.[84] This concentration difference results in a continued diffusive flux and suggests that sediments are a major DOC source releasing 350 Tg C yr–1, which is comparable to the input of DOC from rivers.[85] This estimate is based on calculated diffusive fluxes and does not include resuspension events which also releases DOC [86] and therefore the estimate could be conservative. Also, some studies have shown that geothermal systems and petroleum seepage contribute with pre-aged DOC to the deep ocean basins,[87][88] but consistent global estimates of the overall input are currently lacking. Globally, groundwaters account for an unknown part of the freshwater DOC flux to the oceans.[89] The DOC in groundwater is a mixture of terrestrial, infiltrated marine, and in situ microbially produced material.[90] This flux of DOC to coastal waters could be important, as concentrations in groundwater are generally higher than in coastal seawater,[91] but reliable global estimates are also currently lacking.[7]
Sinks
The main processes that remove DOC from the ocean water column are: (1) Thermal degradation in e.g., submarine hydrothermal systems;[92] (2) bubble coagulation and abiotic flocculation into microparticles [93] or sorption to particles;[94] (3) abiotic degradation via photochemical reactions;[95][96] and (4) biotic degradation by heterotrophic marine prokaryotes.[97] It has been suggested that the combined effects of photochemical and microbial degradation represent the major sinks of DOC.[98][7]
Thermal degradation
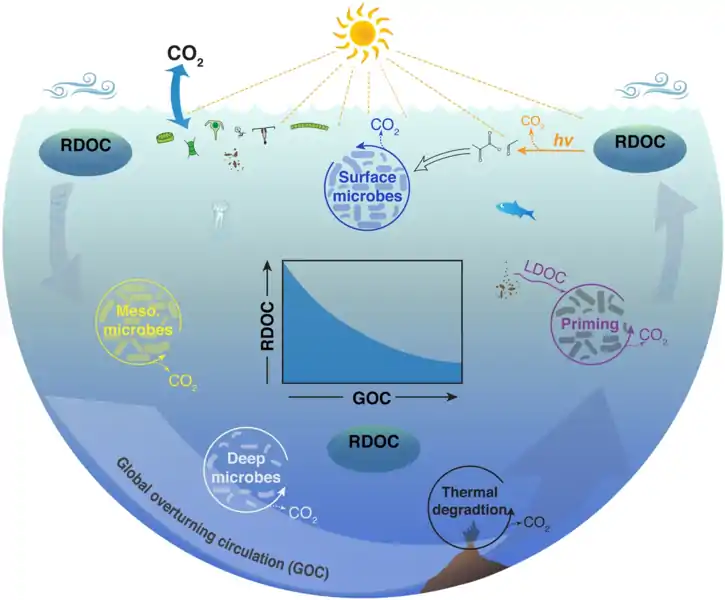 Removal of refractory DOC in the oceanPhytoplankton production and food web dynamics in surface waters release a diverse mixture of dissolved molecules with varying reactivities. Bacteria and archaea utilize labile and semi-labile forms of DOC in surface and mesopelagic waters of the upper ocean, leaving behind a vast reservoir of refractory DOC (RDOC) that persists in the ocean for millennia. The ocean is a patchy environment that harbors a great diversity of microbes and physicochemical processes with the potential to remove refractory DOC when these molecules encounter environmental conditions and microbes that can degrade them. Physical mixing transports refractory DOC throughout the ocean realm and thereby increases the likelihood of its removal. Deep ocean waters can be entrained into hydrothermal circulation and associated DOC can be removed by thermal degradation. Sinking particles from the upper ocean release labile DOC (LDOC) that triggers hot spots of microbial activity and primes the removal of refractory molecules. Mixing of subsurface waters into sunlit waters exposes refractory DOC to warmer temperatures and photochemical processes that can mineralize and transform refractory molecules into simple compounds (e.g., pyruvate, formaldehyde) for rapid microbial utilization. Thus, it appears the lifetime of refractory molecules in the ocean is regulated by the rate of global overturning circulation (GOC). This relationship indicates a slowing of GOC could lead to an increase in the reservoir size of refractory DOC, assuming a constant production rate of refractory DOC (inset panel).[99]
Removal of refractory DOC in the oceanPhytoplankton production and food web dynamics in surface waters release a diverse mixture of dissolved molecules with varying reactivities. Bacteria and archaea utilize labile and semi-labile forms of DOC in surface and mesopelagic waters of the upper ocean, leaving behind a vast reservoir of refractory DOC (RDOC) that persists in the ocean for millennia. The ocean is a patchy environment that harbors a great diversity of microbes and physicochemical processes with the potential to remove refractory DOC when these molecules encounter environmental conditions and microbes that can degrade them. Physical mixing transports refractory DOC throughout the ocean realm and thereby increases the likelihood of its removal. Deep ocean waters can be entrained into hydrothermal circulation and associated DOC can be removed by thermal degradation. Sinking particles from the upper ocean release labile DOC (LDOC) that triggers hot spots of microbial activity and primes the removal of refractory molecules. Mixing of subsurface waters into sunlit waters exposes refractory DOC to warmer temperatures and photochemical processes that can mineralize and transform refractory molecules into simple compounds (e.g., pyruvate, formaldehyde) for rapid microbial utilization. Thus, it appears the lifetime of refractory molecules in the ocean is regulated by the rate of global overturning circulation (GOC). This relationship indicates a slowing of GOC could lead to an increase in the reservoir size of refractory DOC, assuming a constant production rate of refractory DOC (inset panel).[99]
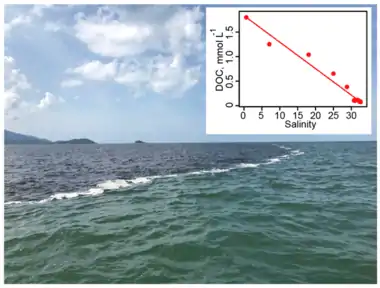
Thermal degradation of DOC has been found at high-temperature hydrothermal ridge-flanks, where outflow DOC concentrations are lower than in the inflow. While the global impact of these processes has not been investigated, current data suggest it is a minor DOC sink.[100] Abiotic DOC flocculation is often observed during rapid (minutes) shifts in salinity when fresh and marine waters mix.[101] Flocculation changes the DOC chemical composition, by removing humic compounds and reducing molecular size, transforming DOC to particulate organic flocs which can sediment and/or be consumed by grazers and filter feeders, but it also stimulates the bacterial degradation of the flocculated DOC.[102] The impacts of flocculation on the removal of DOC from coastal waters are highly variable with some studies suggesting it can remove up to 30% of the DOC pool,[103][104] while others find much lower values (3–6%;[105]). Such differences could be explained by seasonal and system differences in the DOC chemical composition, pH, metallic cation concentration, microbial reactivity, and ionic strength.[101][106][7]
CDOM
The colored fraction of DOC (CDOM) absorbs light in the blue and UV-light range and therefore influences plankton productivity both negatively by absorbing light, that otherwise would be available for photosynthesis, and positively by protecting plankton organisms from harmful UV-light.[107][108] However, as the impact of UV damage and ability to repair is extremely variable, there is no consensus on how UV-light changes might impact overall plankton communities.[109][110] The CDOM absorption of light initiates a complex range of photochemical processes, which can impact nutrient, trace metal and DOC chemical composition, and promote DOC degradation.[111]
Photodegradation
Photodegradation involves the transformation of CDOM into smaller and less colored molecules (e.g., organic acids), or into inorganic carbon (CO, CO2), and nutrient salts (NH+4, HPO2−4).[112][113][114] Therefore, it generally means that photodegradation transforms recalcitrant into labile DOC molecules that can be rapidly used by prokaryotes for biomass production and respiration. However, it can also increase CDOM through the transformation of compounds such as triglycerides, into more complex aromatic compounds,[115][116] which are less degradable by microbes. Moreover, UV radiation can produce e.g., reactive oxygen species, which are harmful to microbes.[117] The impact of photochemical processes on the DOC pool depends also on the chemical composition,[118] with some studies suggesting that recently produced autochthonous DOC becomes less bioavailable while allochthonous DOC becomes more bioavailable to prokaryotes after sunlight exposure, albeit others have found the contrary.[119][120][121] Photochemical reactions are particularly important in coastal waters which receive high loads of terrestrial derived CDOM, with an estimated ∼20–30% of terrestrial DOC being rapidly photodegraded and consumed.[122] Global estimates also suggests that in marine systems photodegradation of DOC produces ∼180 Tg C yr–1 of inorganic carbon, with an additional 100 Tg C yr–1 of DOC made more available to microbial degradation.[123][124] Another attempt at global ocean estimates also suggest that photodegradation (210 Tg C yr–1) is approximately the same as the annual global input of riverine DOC (250 Tg C yr–1;[125]), while others suggest that direct photodegradation exceeds the riverine DOC inputs.[126][127][7]
Recalcitrant DOC
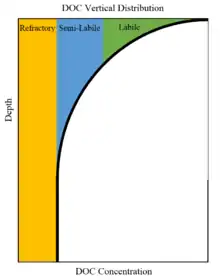
Distribution
More precise measurement techniques developed in the late 1990s have allowed for a good understanding of how dissolved organic carbon is distributed in marine environments both vertically and across the surface.[128] It is now understood that dissolved organic carbon in the ocean spans a range from very labile to very recalcitrant (refractory). The labile dissolved organic carbon is mainly produced by marine organisms and is consumed in the surface ocean, and consists of sugars, proteins, and other compounds that are easily used by marine bacteria.[129] The recalcitrant dissolved organic carbon is evenly spread throughout the water column and consists of high molecular weight and structurally complex compounds that are difficult for marine organisms to use such as the lignin, pollen, or humic acids.[130] Therefore, the observed vertical distribution consists of high concentrations of labile DOC in the upper water column and low concentrations at depth.
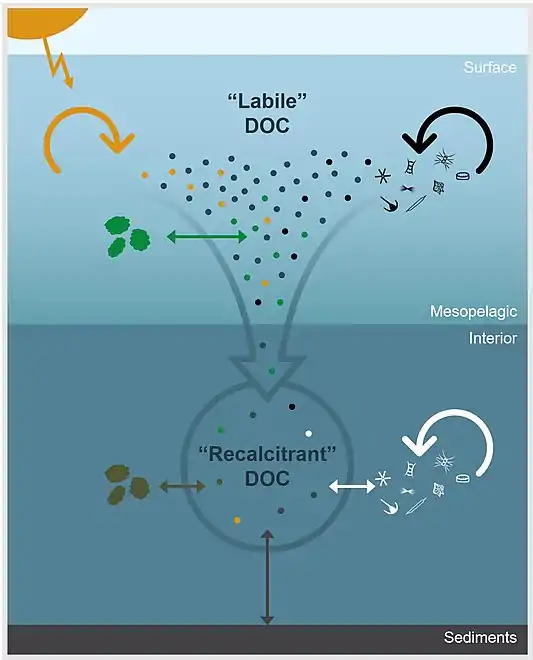 Environmental processes controlling the apparent recalcitrance of oceanic DOCThe dots represent DOC molecules and arrows represent physicochemical and biological processes that impact DOC concentration and molecular composition. In the surface ocean, DOC derived from primary production is rapidly remineralized or transformed through microbial degradation (black arrow), photochemical degradation (yellow arrow), or particle exchange (green arrow). Labile components are removed down the water column and DOC becomes diluted by processes, such as particle exchange (brown arrow), sediment dissolution (gray arrow), and microbial reworking (white arrow), which continue to alter, add, and/or remove molecules from the bulk DOC pool. Thus, the apparent recalcitrance of DOC in the ocean’s interior is an emergent property that is largely controlled by environmental context.[76]
Environmental processes controlling the apparent recalcitrance of oceanic DOCThe dots represent DOC molecules and arrows represent physicochemical and biological processes that impact DOC concentration and molecular composition. In the surface ocean, DOC derived from primary production is rapidly remineralized or transformed through microbial degradation (black arrow), photochemical degradation (yellow arrow), or particle exchange (green arrow). Labile components are removed down the water column and DOC becomes diluted by processes, such as particle exchange (brown arrow), sediment dissolution (gray arrow), and microbial reworking (white arrow), which continue to alter, add, and/or remove molecules from the bulk DOC pool. Thus, the apparent recalcitrance of DOC in the ocean’s interior is an emergent property that is largely controlled by environmental context.[76]
In addition to vertical distributions, horizontal distributions have been modeled and sampled as well.[131] In the surface ocean at a depth of 30 meters, the higher dissolved organic carbon concentrations are found in the South Pacific Gyre, the South Atlantic Gyre, and the Indian Ocean. At a depth of 3,000 meters, highest concentrations are in the North Atlantic Deep Water where dissolved organic carbon from the high concentration surface ocean is removed to depth. While in the northern Indian Ocean high DOC is observed due to high fresh water flux and sediments. Since the time scales of horizontal motion along the ocean bottom are in the thousands of years, the refractory dissolved organic carbon is slowly consumed on its way from the North Atlantic and reaches a minimum in the North Pacific.
As emergent
Dissolved organic matter is a heterogeneous pool of thousands, likely millions, of organic compounds. These compounds differ not only in composition and concentration (from pM to μM), but also originate from various organisms (phytoplankton, zooplankton, and bacteria) and environments (terrestrial vegetation and soils, coastal fringe ecosystems) and may have been produced recently or thousands of years ago. Moreover, even organic compounds deriving from the same source and of the same age may have been subjected to different processing histories prior to accumulating within the same pool of DOM.[76]
Interior ocean DOM is a highly modified fraction that remains after years of exposure to sunlight, utilization by heterotrophs, flocculation and coagulation, and interaction with particles. Many of these processes within the DOM pool are compound- or class-specific. For example, condensed aromatic compounds are highly photosensitive,[132] whereas proteins, carbohydrates, and their monomers are readily taken up by bacteria.[133][134][135] Microbes and other consumers are selective in the type of DOM they utilize and typically prefer certain organic compounds over others. Consequently, DOM becomes less reactive as it is continually reworked. Said another way, the DOM pool becomes less labile and more refractory with degradation. As it is reworked, organic compounds are continually being added to the bulk DOM pool by physical mixing, exchange with particles, and/or production of organic molecules by the consumer community.[136][137][138][139] As such, the compositional changes that occur during degradation are more complex than the simple removal of more labile components and resultant accumulation of remaining, less labile compounds.[76]
Dissolved organic matter recalcitrance (i.e., its overall reactivity toward degradation and/or utilization) is therefore an emergent property. The perception of DOM recalcitrance changes during organic matter degradation and in conjunction with any other process that removes or adds organic compounds to the DOM pool under consideration.[76]
Interaction with metals
DOC also facilitates the transport of metals in aquatic systems. Metals form complexes with DOC, enhancing metal solubility while also reducing metal bioavailability.
DOM isolation and analysis
DOM is found in low concentrations in nature for direct analysis with NMR or MS. Moreover, DOM samples often contain high concentrations of inorganic salts that are incompatible with such techniques.[140] Therefore, it is necessary a concentration and isolation step of the sample.[141][142] The most used isolation techniques are ultrafiltration, reverse osmosis and solid-phase extraction.[143] Among them solid-phase extraction is considered as the cheapest and easiest technique[144]
References
- Roshan, S. and DeVries, T. (2017) "Efficient dissolved organic carbon production and export in the oligotrophic ocean". Nature Communications, 8(1): 1–8. doi:10.1038/s41467-017-02227-3.
- "Organic Carbon". Bio-geochemical Methods. Retrieved 2018-11-27.
- Kenny, Jonathan E.; Bida, Morgan; Pagano, Todd (October 2014). "Trends in Levels of Allochthonous Dissolved Organic Carbon in Natural Water: A Review of Potential Mechanisms under a Changing Climate". Water. 6 (10): 2862–2897. doi:10.3390/w6102862.
- Moody, C.S. and Worrall, F. (2017) "Modeling rates of DOC degradation using DOM composition and hydroclimatic variables". Journal of Geophysical Research: Biogeosciences, 122(5): 1175–1191. doi:10.1002/2016JG003493.
- Hedges, John I. (3 December 1991). "Global biogeochemical cycles: progress and problems" (PDF). Marine Chemistry. 39 (1–3): 67–93. doi:10.1016/0304-4203(92)90096-s.
- Kritzberg, Emma S.; Cole, Jonathan J.; Pace, Michael L.; Granéli, Wilhelm; Bade, Darren L. (March 2004). "Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake 13C addition experiments" (PDF). Limnology and Oceanography. 49 (2): 588–596. Bibcode:2004LimOc..49..588K. doi:10.4319/lo.2004.49.2.0588. ISSN 0024-3590.
- Lønborg, C., Carreira, C., Jickells, T. and Álvarez-Salgado, X.A. (2020) "Impacts of global change on ocean dissolved organic carbon (DOC) cycling". Frontiers in Marine Science, 7: 466. doi:10.3389/fmars.2020.00466.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Monroy, P., Hernández-García, E., Rossi, V. and López, C. (2017) "Modeling the dynamical sinking of biogenic particles in oceanic flow". Nonlinear Processes in Geophysics, 24(2): 293–305. doi:10.5194/npg-24-293-2017.
 Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License. - Simon, M., Grossart, H., Schweitzer, B. and Ploug, H. (2002) "Microbial ecology of organic aggregates in aquatic ecosystems". Aquatic microbial ecology, 28: 175–211. doi:10.3354/ame028175.
- Kirchman, David L.; Suzuki, Yoshimi; Garside, Christopher; Ducklow, Hugh W. (15 August 1991). "High turnover rates of dissolved organic carbon during a spring phytoplankton bloom". Nature. 352 (6336): 612–614. Bibcode:1991Natur.352..612K. doi:10.1038/352612a0. S2CID 4285758.
- Jaeckle, W.B.; Manahan, D.T. (1989). "Feeding by a "nonfeeding" larva: uptake of dissolved amino acids from seawater by lecithotrophic larvae of the gastropod Haliotis rufescens". Marine Biology. 103: 87–94. doi:10.1007/BF00391067. S2CID 84541307.
- Cheremisinoff, Nicholas; Davletshin, Anton (2015). "Hydraulic Fracturing Operations: Handbook of Environmental Management Practices". Environmental Management. ISBN 9781119099994.
- Elser, Stephen (2014). "Brown Water: The Ecological and Economic Implications of Increased Dissolved Organic Carbon in Lakes". Cite journal requires
|journal=(help) - Wu, Qing; Zhao, Xin-Hua; Wang, Xiao-Dan (2008). "Relationship Between Heterotrophic Bacteria and Some Physical and Chemical Parameters in a Northern City's Drinking Water Distribution Networks of China". 2008 2nd International Conference on Bioinformatics and Biomedical Engineering. pp. 4713–4716. doi:10.1109/ICBBE.2008.336. ISBN 978-1-4244-1747-6. S2CID 24876521.
- "Dissolved Organic Carbon (DOC)".
- Narayana, P.S.; Varalakshmi, D; Pullaiah, T; Sambasiva Rao, K.R.S. (2018). Research Methodology in Zoology. p. 225. ISBN 9789388172400.
- "Whatman® glass microfiber filters, Grade GF/F". Merck.
- Knap, A. Michaels; A. Close; A. Ducklow; H. Dickson, A. (1994). Protocols for the Joint Global Ocean Flux studies (JGOFS) core measurements. JGOFS.
- Cauwet G (2002) "DOM in the Coastal Zone". In: Hansell D and Carlson C (Eds.) Biogeochemistry of Marine Dissolved Organic Matter, pages 579–610, Elsevier. ISBN 9780080500119.
- Tremblay, L. and Benner, R. (2006) "Microbial contributions to N-immobilization and organic matter preservation in decaying plant detritus". Geochimica et Cosmochimica Acta, 70(1): 133–146. doi:10.1016/j.gca.2005.08.024.
- Jiao, N., Herndl, G.J., Hansell, D.A., Benner, R., Kattner, G., Wilhelm, S.W., Kirchman, D.L., Weinbauer, M.G., Luo, T., Chen, F. and Azam, F. (2010) "Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean". Nature Reviews: Microbiology, 8(8): 593–599. doi:10.1038/nrmicro2386.
- Lee, S.A., Kim, T.H. and Kim, G. (2020) "Tracing terrestrial versus marine sources of dissolved organic carbon in a coastal bay using stable carbon isotopes". Biogeosciences, 17(1). doi:10.5194/bg-17-135-2020.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Vahatalo, A. V., Aarnos, H., and Mantyniemi, S. (2010). Biodegradability continuum and biodegradation kinetics of natural organic matter described by the beta distribution. Biogeochemistry 100, 227–240. doi: 10.1007/s10533-010-9419-4
- Hansell, D. A. (2013). Recalcitrant dissolved organic carbon fractions. Ann. Rev. Mar. Sci. 5, 421–445. doi: 10.1146/annurev-marine-120710-100757
- Amon, R. M. W., and Benner, R. (1996). Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41, 41–51. doi: 10.4319/lo.1996.41.1.0041
- Benner, R., and Amon, R. M. (2015). The size-reactivity continuum of major bioelements in the ocean. Ann. Rev. Mar. Sci. 7, 185–205. doi: 10.1146/annurev-marine-010213-135126
- Thingstad, T. F., Havskum, H., Kaas, H., Nielsen, T. G., Riemann, B., Lefevre, D., et al. (1999). Bacteria-protist interactions and organic matter degradation under P-limited conditions: analysis of an enclosure experiment using a simple model. Limnol. Oceanogr. 44, 62–79. doi: 10.4319/lo.1999.44.1.0062
- Del-Giorgio, P., and Davies, J. (2003). “Patterns of dissolved organic matter lability and consumption across aquatic ecosystems,” in Aquatic Ecosystems: Interactivity of Dissolved Organic Matter, eds S. E. G. Findlay and R. L. Sinsabaugh (San Diego, CA: Academic Press), 399–424. doi: 10.1016/B978-012256371-3/50018-4
- Bianchi, T. S. (2011). The role of terrestrially derived organic carbon in the coastal ocean: a changing paradigm and the priming effect. Proc. Natl. Acad. Sci. U.S.A. 108, 19473–19481. doi: 10.1073/pnas.1017982108
- Kattner, G., Simon, M., and Koch, B. P. (2011). “Molecular characterization of dissolved organic matter and constraints for prokaryotic utilization,” in Microbial Carbon Pump in the Ocean, eds N. Jiao, F. Azam, and S. Sansers (Washington, DC: Science/AAAS).
- Keil, R. G., and Mayer, L. M. (2014). “Mineral matrices and organic matter,” in Treatise on Geochemistry, 2nd Edn, eds H. Holland and K. Turekian (Oxford: Elsevier), 337–359. doi: 10.1016/B978-0-08-095975-7.01024-X
- Bianchi, T. S., Cui, X., Blair, N. E., Burdige, D. J., Eglinton, T. I., and Galy, V. (2018). Centers of organic carbon burial and oxidation at the land-ocean interface. Org. Geochem. 115, 138–155. doi: 10.1016/j.orggeochem.2017.09.008
- Ward, N. D., Keil, R. G., Medeiros, P. M., Brito, D. C., Cunha, A. C., Dittmar, T., et al. (2013). Degradation of terrestrially derived macromolecules in the Amazon River. Nat. Geosci. 6, 530–533. doi: 10.1038/ngeo1817
- Myers-Pigg, A. N., Louchouarn, P., Amon, R. M. W., Prokushkin, A., Pierce, K., and Rubtsov, A. (2015). Labile pyrogenic dissolved organic carbon in major Siberian Arctic rivers: implications for wildfire-stream metabolic linkages. Geophys. Res. Lett. 42, 377–385. doi: 10.1002/2014GL062762
- Gmach, M.R., Cherubin, M.R., Kaiser, K. and Cerri, C.E.P. (2020) "Processes that influence dissolved organic matter in the soil: a review". Scientia Agricola, 77(3). doi:10.1590/1678-992x-2018-0164.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Shen, Y., Chapelle, F.H., Strom, E.W. and Benner, R. (2015) "Origins and bioavailability of dissolved organic matter in groundwater". Biogeochemistry, 122(1): 61–78. doi:10.1038/s41467-019-11394-4.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Reitsema, R.E., Meire, P. and Schoelynck, J. (2018) "The future of freshwater macrophytes in a changing world: dissolved organic carbon quantity and quality and its interactions with macrophytes". Frontiers in plant science, 9: 629. doi:10.3389/fpls.2018.00629.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Thomas, J. D. (1997). The role of dissolved organic matter, particularly free amino acids and humic substances, in freshwater ecosystems. Freshw. Biol. 38, 1–36. doi: 10.1046/j.1365-2427.1997.00206.x
- Cole, J. J., Prairie, Y. T., Caraco, N. F., McDowell, W. H., Tranvik, L. J., Striegl, R. G., et al. (2007). Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 172–185. doi: 10.1007/s10021-006-9013-8
- Raymond, P. A., Hartmann, J., Lauerwald, R., Sobek, S., McDonald, C., Hoover, M., et al. (2013). Global carbon dioxide emissions from inland waters. Nature 503, 355–359. doi: 10.1038/nature12760
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. 2000. Controls on the dynamics of dissolved organic matter in soils: a review. Soil Science 165: 277–304.
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. 1997. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 79: 117–161.
- Saidy, A.R.; Smernik, R.J.; Baldock, J.A.; Kaiser, K.; Sanderman, J. 2015. Microbial degradation of organic carbon sorbed to phyllosilicate clays with and without hydrous iron oxide coating. European Journal of Soil Science 66: 83–94.
- Kaiser, K.; Guggenberger, G. 2007. Sorptive stabilization of organic matter by microporous goethite: sorption into small pores vs. surface complexation. European Journal of Soil Science 58: 45–59.
- Veum, K.S.; Goyne, K.W.; Motavalli, P.P.; Udawatta, R.P. 2009. Runoff and dissolved organic carbon loss from a paired-watershed study of three adjacent agricultural Watersheds. Agriculture, Ecosystems & Environment 130: 115–122.
- Veum, K.S.; Goyne, K.W.; Motavalli, P.P.; Udawatta, R.P. 2009. Runoff and dissolved organic carbon loss from a paired-watershed study of three adjacent agricultural Watersheds. Agriculture, Ecosystems & Environment 130: 115–122.
- Sparling, G.; Chibnall, E.; Pronger, J.; Rutledge, S.; Wall, A.; Campbell, D.; Schipper, L. 2016. Estimates of annual leaching losses of dissolved organic carbon from pastures on Allophanic soils grazed by dairy cattle, Waikato, New Zealand. New Zealand Journal of Agricultural Research 59: 32–49.
- Sobek, S., Tranvik, L. J., Prairie, Y. T., Kortelainen, P., and Cole, J. J. (2007). Patterns and regulation of dissolved organic carbon: an analysis of 7,500 widely distributed lakes. Limnol. Oceanogr. 52, 1208–1219. doi: 10.4319/lo.2007.52.3.1208
- Stumm, W., and Morgan, J. J. (1996). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. Environmental Science and Technology. New York: John Wiley & Sons, Inc.
- Madsen, T. V., and Sand-Jensen, K. (1991). Photosynthetic carbon assimilation in aquatic macrophytes. Aquat. Bot. 41, 5–40. doi: 10.1016/0304-3770(91)90037-6
- Regnier, P., Friedlingstein, P., Ciais, P., Mackenzie, F. T., Gruber, N., Janssens, I. A., et al. (2013). Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci. 6, 597–607. doi: 10.1038/ngeo1830
- Luyssaert, S., Abril, G., Andres, R., Bastviken, D., Bellassen, V., Bergamaschi, P., et al. (2012). The European land and inland water CO2, CO, CH4 and N2O balance between 2001 and 2005. Biogeosciences 9, 3357–3380. doi: 10.5194/bg-9-3357-2012
- Kawasaki, N., and Benner, R. (2006). Bacterial release of dissolved organic matter during cell growth and decline: molecular origin and composition. Limnol. Oceanogr. 51, 2170–2180. doi: 10.4319/lo.2006.51.5.2170
- Lønborg, C., Álvarez-Salgado, X. A., Davidson, K., and Miller, A. E. J. (2009). Production of bioavailable and refractory dissolved organic matter by coastal heterotrophic microbial populations. Estuar. Coast. Shelf Sci. 82, 682–688. doi: 10.1016/j.ecss.2009.02.026
- Wada, S., Aoki, M. N., Tsuchiya, Y., Sato, T., Shinagawa, H., and Hama, T. (2007). Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan. J. Exp. Mar. Biol. Ecol. 349, 344–358. doi: 10.1016/j.jembe.2007.05.024
- Willey, J. D., Kieber, R. J., Eyman, M. S. Jr., and Brooks Avery, G. (2000). Rainwater dissolved organic carbon concentrations and global flux. Glob. Biogeochem. Cycles 14, 139–148. doi: 10.1029/1999GB900036
- Raymond, P. A., and Spencer, R. G. M. (2015). “Riverine DOM,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (Amsterdam: Elsevier), 509–533. doi: 10.1016/B978-0-12-405940-5.00011-X
- Dachs, J., and Méjanelle, L. (2010). Organic pollutants in coastal waters, sediments, and biota: a relevant driver for ecosystems during the anthropocene? Estuarines Coasts 33, 1–14. doi: 10.1007/s12237-009-9255-8
- Raymond, P. A., and Spencer, R. G. M. (2015). “Riverine DOM,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansell and C. A. Carlson (Amsterdam: Elsevier), 509–533. doi: 10.1016/B978-0-12-405940-5.00011-X
- Karl, D. M., Hebel, D. V., Bjorkman, K., and Letelier, R. M. (1998). The role of dissolved organic matter release in the productivity of the oligotrophic north Pacific Ocean. Limnol. Oceanogr. 43, 1270–1286. doi: 10.4319/lo.1998.43.6.1270
- Wetz, M. S., and Wheeler, P. A. (2007). Release of dissolved organic matter by coastal diatoms. Limnol. Oceanogr. 52, 798–807. doi: 10.4319/lo.2007.52.2.0798
- Thornton, D. C. O. (2014). Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. Eur. J. Phycol. 49, 20–46. doi: 10.1080/09670262.2013.875596
- Boekell, W. H. M. V., Hansen, F. C., Riegman, R., and Bak, R. P. M. (1992). Lysis-induced decline of a Phaeocystis spring bloom and coupling with the microbial foodweb. Mar. Ecol. Prog. Ser. 81, 269–276. doi: 10.3354/meps081269
- Hygum, B. H., Petersen, J. W., and Søndergaard, M. (1997). Dissolved organic carbon released by zooplankton grazing activity- a high quality substrate pool for bacteria. J. Plankton Res. 19, 97–111. doi: 10.1093/plankt/19.1.97
- Lampert, W. (1978). Release of dissolved organic carbon by grazing zooplankton. Limnol. Oceanogr. 23, 831–834. doi: 10.4319/lo.1978.23.4.0831
- Jumars, P. A., Penry, D. L., Baross, J. A., and Perry, M. J. (1989). Closing the microbial loop: dissolved carbon pathway to heterotrophic bacteria from incomplete ingestion, digestion and absorption in animals. Deep Sea Res. 36, 483–495. doi: 10.1016/0198-0149(89)90001-0
- Iturriaga, R., and Zsolnay, A. (1981). Transformation of some dissolved organic compounds by a natural heterotrophic population. Mar. Biol. 62, 125–129. doi: 10.1007/BF00388174
- Ogawa, H., Amagai, Y., Koike, I., Kaiser, K., and Benner, R. (2001). Production of refractory dissolved organic matter by bacteria. Science 292, 917–920. doi: 10.1126/science.1057627
- Kawasaki, N., and Benner, R. (2006). Bacterial release of dissolved organic matter during cell growth and decline: molecular origin and composition. Limnol. Oceanogr. 51, 2170–2180. doi: 10.4319/lo.2006.51.5.2170
- McCarthy, M., Pratum, T., Hedges, J., and Benner, R. (1997). Chemical composition of dissolved organic nitrogen in the ocean. Nature 390, 150–154. doi: 10.1038/36535
- Suttle, C. A. (2005). Viruses in the sea. Nature 437, 356–361. doi: 10.1038/nature04160
- Weinbauer, M. A. G. (2004). Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28, 127–181. doi: 10.1016/j.femsre.2003.08.001
- Lønborg, C., Middelboe, M., and Brussaard, C. P. D. (2013). Viral lysis of Micromonas pusilla: impacts on dissolved organic matter production and composition. Biogeochemistry 116, 231–240. doi: 10.1007/s10533-013-9853-1
- Krabberød, AK; Bjorbækmo, MFM; Shalchian-Tabrizi, K.; Logares, R. (2017). "Exploring the oceanic microeukaryotic interactome with metaomics approaches". Aquatic Microbial Ecology. 79: 1–12. doi:10.3354/ame01811.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - Delong, Edward F.; Karl, David M. (2005). "Genomic perspectives in microbial oceanography". Nature. 437 (7057): 336–342. doi:10.1038/nature04157. PMID 16163343. S2CID 4400950.
- Wagner, S., Schubotz, F., Kaiser, K., Hallmann, C., Waska, H., Rossel, P.E., Hansman, R., Elvert, M., Middelburg, J.J., Engel, A. and Blattmann, T.M. (2020) "Soothsaying DOM: A current perspective on the future of oceanic dissolved organic carbon". Frontiers in Marine Science, 7:341. doi:10.3389/fmars.2020.00341.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Brilinsky, M. (1977). Release of dissolved organic matter by some marine macrophytes. Mar. Biol. 39, 213–220. doi: 10.1007/BF00390995
- Pregnall, A. M. (1983). Release of dissolved organic carbon from the estuarine intertidal macroalga Enteromorpha prolifera. Mar. Biol. 73, 37–42. doi: 10.1007/BF00396283
- Penhale, P. A., and Smith, W. O. (1977). Excretion of dissolved organic carbon by eelgrass (Zostera marina) and its epiphytes. Limnol. Oceanogr. 22, 400–407. doi: 10.4319/lo.1977.22.3.0400
- Barrón, C., and Duarte, C. M. (2015). Dissolved organic carbon pools and export from the coastal ocean. Glob. Biogeochem. Cycles 29, 1725–1738. doi: 10.1002/2014GB005056
- Barrón, C., and Duarte, C. M. (2015). Dissolved organic carbon pools and export from the coastal ocean. Glob. Biogeochem. Cycles 29, 1725–1738. doi: 10.1002/2014GB005056
- Martin, P., Cherukuru, N., Tan, A.S., Sanwlani, N., Mujahid, A. and Müller, M.(2018) "Distribution and cycling of terrigenous dissolved organic carbon in peatland-draining rivers and coastal waters of Sarawak, Borneo", Biogeosciences, 15(2): 6847–6865. doi:10.5194/bg-15-6847-2018.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Hewson, I., O’neil, J. M., Fuhrman, J. A., and Dennison, W. C. (2001). Virus-like particle distribution and abundance in sediments and overlying waters along eutrophication gradients in two subtropical estuaries. Limnol. Oceanogr. 46, 1734–1746. doi: 10.4319/lo.2001.46.7.1734
- Burdige, D. J., and Gardner, K. G. (1998). Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Mar. Chem. 62, 45–64. doi: 10.1016/S0304-4203(98)00035-8
- Burdige, D. J., and Komada, T. (2014). “Sediment pore waters,” in Biogeochemistry of Marine Dissolved Organic Matter, eds D. A. Hansen and C. A. Carlson (Cambridge, MA: Academic Press), 535–577. doi: 10.1016/B978-0-12-405940-5.00012-1
- Komada, T., and Reimers, C. E. (2001). Resuspension-induced partitioning of organic carbon between solid and solution phases from a river–ocean transition. Mar. Chem. 76, 155–174. doi: 10.1016/S0304-4203(01)00055-X
- Dittmar, T., and Koch, B. P. (2006). Thermogenic organic matter dissolved in the abyssal ocean. Mar. Chem. 102, 208–217. doi: 10.1016/j.marchem.2006.04.003
- Dittmar, T., and Paeng, J. (2009). A heat-induced molecular signature in marine dissolved organic matter. Nat. Geosci. 2, 175–179. doi: 10.1038/ngeo440
- Burnett, W. C., Aggarwal, P. K., Aureli, A., Bokuniewicz, H., Cable, J. E., Charette, M. A., et al. (2006). Quantifying submarine groundwater discharge in the coastal zone via multiple methods. Sci. Total Environ. 367, 498–543. doi: 10.1016/j.scitotenv.2006.05.009
- Longnecker, K., and Kujawinski, E. B. (2011). Composition of dissolved organic matter in groundwater. Geochim. Cosmochim. Acta 75, 2752–2761. doi: 10.1016/j.gca.2011.02.020
- Webb, J. R., Santos, I. R., Maher, D. T., Tait, D. R., Cyronak, T., Sadat-Noori, M., et al. (2019). Groundwater as a source of dissolved organic matter to coastal waters: insights from radon and CDOM observations in 12 shallow coastal systems. Limnol. Oceanogr. 64, 182–196. doi: 10.1002/lno.11028
- Lang, S. Q., Butterfield, D. A., Lilley, M. D., Paul Johnson, H., and Hedges, J. I. (2006). Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim. Cosmochim. Acta 70, 3830–3842. doi: 10.1016/j.gca.2006.04.031
- Kerner, M., Hohenberg, H., Ertl, S., Reckermann, M., and Spitzy, A. (2003). Self-organization of dissolved organic matter tomicelle-like microparticles in river water. Nature 422, 150–154. doi: 10.1038/nature01469
- Chin, W. C., Orellana, M. V., and Verdugo, P. (1998). Spontaneous assembly of marine dissolved organic matter into polymer gels. Nature 391, 568–572. doi: 10.1038/35345
- Moran, M. A., and Zepp, R. G. (1997). Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 42, 1307–1316. doi: 10.4319/lo.1997.42.6.1307
- Mopper, K., Kieber, D. J., and Stubbins, A. (2015). “Marine photochemistry of organic matter,” in Biogeochemistry of Marine Dissolved Organic Matter, eds C. A. Carlson and D. A. Hansell (Amsterdam: Elsevier), 389–450. doi: 10.1016/B978-0-12-405940-5.00008-X
- Lønborg, C., and Álvarez-Salgado, X. A. (2012). Recycling versus export of bioavailable dissolved organic matter in the coastal ocean and efficiency of the continental shelf pump. Glob. Biogeochem. Cycles 26:GB3018. doi: 10.1029/2012GB004353
- Carlson, C. A., and Hansell, D. A. (2015). “DOM sources, sinks, reactivity, and budgets,” in Biogeochemistry of Marine Dissolved Organic Matter, eds C. A. Carlson and D. A. Hansell (San Diego, CA: Academic Press), 65–126. doi: 10.1016/B978-0-12-405940-5.00003-0
- Shen, Y. and Benner, R. (2018) "Mixing it up in the ocean carbon cycle and the removal of refractory dissolved organic carbon". Scientific reports, 8(1): 1–9. doi:10.1038/s41598-018-20857-5.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Lang, S. Q., Butterfield, D. A., Lilley, M. D., Paul Johnson, H., and Hedges, J. I. (2006). Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim. Cosmochim. Acta 70, 3830–3842. doi: 10.1016/j.gca.2006.04.031
- Sholkovitz, E. R. (1976). Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim. Cosmochim. Acta 40, 831–845. doi: 10.1016/0016-7037(76)90035-1
- Tranvik, L. J., and Sieburth, J. M. (1989). Effects of flocculated humic matter on free and attached pelagic microorganisms. Limnol. Oceanogr. 34, 688–699. doi: 10.4319/lo.1989.34.4.0688
- Mulholland, P. J. (1981). Formation of Particulate Organic Carbon in Water from a Southeastern Swamp-Stream. Limnol. Oceanogr. 26, 790–795. doi: 10.4319/lo.1981.26.4.0790
- Powell, R. T., Landing, W. M., and Bauer, J. E. (1996). Colloidal trace metals, organic carbon and nitrogen in a southeastern U.S. estuary. Mar. Chem. 55, 165–176. doi: 10.1016/S0304-4203(96)00054-0
- Sholkovitz, E. R., Boyle, E. A., and Price, N. B. (1978). The removal of dissolved humic acids and iron during estuarine mixing. Earth Planet. Sci. Lett. 40, 130–136. doi: 10.1016/0012-821X(78)90082-1
- Volk, C., Bell, K., Ibrahim, E., Verges, D., Amy, G., and Lechevallier, M. (2000). Impact of enhanced and optimized coagulation on removal of organic matter and its biodegradable fraction in drinking water. Water Res. 34, 3247–3257. doi: 10.1016/S0043-1354(00)00033-6
- Williamson, C. E., Stemberger, R. S., Morris, D. P., Frost, T. A., and Paulsen, S. G. (1996). Ultraviolet radiation in North American lakes: attenuation estimates from DOC measurements and implications for plankton communities. Limnol. Oceanogr. 41, 1024–1034. doi: 10.4319/lo.1996.41.5.1024
- Williamson, C. E., Overholt, E. P., Pilla, R. M., Leach, T. H., Brentrup, J. A., Knoll, L. B., et al. (2015). Ecological consequences of longterm browning in lakes. Sci. Rep. 5:18666. doi: 10.1038/srep18666
- Jeffrey, W. H., Aas, P., Lyons, M. M., Coffin, R. B., Pledger, R. J., and Mitchell, D. L. (1996). Ambient solar radiation-induced photodamage in marine bacterioplankton. Photochem. Photobiol. 64, 419–427. doi: 10.1111/j.1751-1097.1996.tb03086.x
- Rhode, S. C., Pawlowski, M., and Tollrian, R. (2001). The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature 412, 69–72. doi: 10.1038/35083567
- Mopper, K., Kieber, D. J., and Stubbins, A. (2015). “Marine photochemistry of organic matter,” in Biogeochemistry of Marine Dissolved Organic Matter, eds C. A. Carlson and D. A. Hansell (Amsterdam: Elsevier), 389–450. doi: 10.1016/B978-0-12-405940-5.00008-X
- Miller, W. L., and Zepp, R. G. (1995). Photochemical production of dissolved inorganic carbon from terrestrial organic matter: significance of the oceanic organic carbon cycle. Geophys. Res. Lett. 22, 417–420. doi: 10.1029/94GL03344
- Moran, M. A., and Zepp, R. G. (1997). Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 42, 1307–1316. doi: 10.4319/lo.1997.42.6.1307
- Moran, M. A., Sheldon, W. M., and Zepp, R. G. (2000). Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol. Oceanogr. 45, 1254–1264. doi: 10.4319/lo.2000.45.6.1254
- Kieber, R. J., Hydro, L. H., and Seaton, P. J. (1997). Photooxidation of triglycerides and fatty acids in seawater: implication toward the formation of marine humic substances. Limnol. Oceanogr. 42, 1454–1462. doi: 10.4319/lo.1997.42.6.1454
- Berto, S., Laurentiis, E. D., Tota, T., Chiavazza, E., Daniele, P. G., Minella, M., et al. (2016). Properties of the humic-like material arising from the phototransformation of L-tyrosine. Sci. Total Environ. 546, 434–444. doi: 10.1016/j.scitotenv.2015.12.047
- Hudson, J. J., Dillon, P. J., and Somers, K. M. (2003). Long-term patterns in dissolved organic carbon in boreal lakes: the role of incident radiation, precipitation, air temperature, southern oscillation and acid deposition. Hydrol. Earth Syst. Sci. 7, 390–398. doi: 10.5194/hess-7-390-2003
- Benner, R., Benitez-Nelson, B., Kaiser, K., and Amon, R. M. W. (2004). Export of young terrigenous dissolved organic carbon from rivers to the Arctic Ocean. Geophys. Res. Lett. 31:L05305. doi: 10.1029/2003GL019251
- Obernosterer, I., and Herndl, G. J. (1995). Phytoplankton extracellular release and bacterial growth: dependence on the inorganic N:P ratio. Mar. Ecol. Prog. Ser. 116, 247–257. doi: 10.3354/meps116247
- Benner, R., and Ziegler, S. (1999). “Do photochemical transformations of dissolved organic matter produce biorefractory as well as bioreactive substrates?” in Proceedings of the 8th International Symposium on Microbial Ecology, eds C. R. Bell, M. Brylinsky, and P. Johnson-Green (Port Aransas, TX: University of Texas at Austin).
- Sulzberger, B., and Durisch-Kaiser, E. (2009). Chemical characterization of dissolved organic matter (DOM): a prerequisite for understanding UV-induced changes of DOM absorption properties and bioavailability. Aquat. Sci. 71, 104–126. doi: 10.1007/s00027-008-8082-5
- Miller, W. L., and Moran, M. A. (1997). Interaction of photochemical and microbial processes in the degradation of refractory dissolved organic matter from a coastal marine environment. Limnol. Oceanogr. 42, 1317–1324. doi: 10.4319/lo.1997.42.6.1317
- Moran, M. A., and Zepp, R. G. (1997). Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 42, 1307–1316. doi: 10.4319/lo.1997.42.6.1307
- Stubbins, A., Uher, G., Law, C. S., Mopper, K., Robinson, C., and Upstill-Goddard, R. C. (2006). Open-ocean carbon monoxide photoproduction. Deep Sea Res. II Top. Stud. Oceanogr. 53, 1695–1705. doi: 10.1016/j.dsr2.2006.05.011
- Miller, W. L., Moran, M. A., Sheldon, W. M., Zepp, R. G., and Opsahl, S. (2002). Determination of apparent quantum yield spectra for the formation of biologically labile photoproducts. Limnol. Oceanogr. 47, 343–352. doi: 10.4319/lo.2002.47.2.0343
- Andrews, S. S., and Zafiriou, O. C. (2000). Photochemical oxygen consumption in marine waters: a Major soink for colored dissolved organic matter? Limnol. Oceanogr. 45, 267–277. doi: 10.4319/lo.2000.45.2.0267
- Wang, X.-C., Chen, R. F., and Gardner, G. B. (2004). Sources and transport of dissolved and particulate organic carbon in the Mississippi River estuary and adjacent coastal waters of the northern Gulf of Mexico. Mar. Chem. 89, 241–256. doi: 10.1016/j.marchem.2004.02.014
- Sharp, Jonathan H. (6 August 1996). "Marine dissolved organic carbon: Are the older values correct?". Marine Chemistry. 56 (3–4): 265–277. doi:10.1016/S0304-4203(96)00075-8.
- Sondergaard, Morten; Mathias Middelboe (9 March 1995). "A cross-system analysis of labile dissolved organic carbon" (PDF). Marine Ecology Progress Series. 118: 283–294. Bibcode:1995MEPS..118..283S. doi:10.3354/meps118283.
- Gruber, David F.; Jean-Paul Simjouw; Sybil P. Seitzinger; Gary L. Taghon (June 2006). "Dynamics and Characterization of Refractory Dissolved Organic Matter Produced by a Pure Bacterial Culture in an Experimental Predator-Prey System". Applied and Environmental Microbiology. 72 (6): 4184–4191. doi:10.1128/AEM.02882-05. PMC 1489638. PMID 16751530.
- Hansell, Dennis A.; Craig A. Carlson; Daniel J. Repeta; Reiner Schlitzer (2009). "Dissolved Organic Matter in the Ocean: A Controversy Stimulates New Insights". Oceanography. 22 (4): 202–211. doi:10.5670/oceanog.2009.109. hdl:1912/3183.
- Stubbins, A., Niggemann, J., and Dittmar, T. (2012). Photo-lability of deep ocean dissolved black carbon. Biogeosciences 9, 1661–1670. doi: 10.5194/bg-9-1661-2012
- Hodson, R. E., Maccubbin, A. E., and Pomeroy, L. R. (1981). Dissolved adenosine triphosphate utilization by free-living and attached bacterioplankton. Mar. Biol. 64, 43–51. doi: 10.1007/bf00394079
- Hollibaugh, J. T., and Azam, F. (1983). Microbial degradation of dissolved proteins in seawater. Limnol. Oceanogr. 28, 1104–1116. doi: 10.4319/lo.1983.28.6.1104
- Ferguson, R. L., and Sunda, W. G. (1984). Utilization of amino acids by planktonic marine bacteria: importance of clean technique and low substrate additions. Limnol. Oceanogr. 29, 258–274. doi: 10.4319/lo.1984.29.2.0258
- Ogawa, H., Amagai, Y., Kioke, I., Kaiser, K., and Benner, R. (2001). Production of refractory dissolved organic matter by bacteria. Science 292, 917–920. doi: 10.1126/science.1057627
- Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., et al. (2010). Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 8, 593–599. doi: 10.1038/nrmicro2386
- Kaiser, K., and Benner, R. (2008). Major bacterial contribution to the ocean reservoir of detrital organic carbon and nitrogen. Limnol. Oceanogr. 53, 99–112. doi: 10.4319/lo.2008.53.1.0099
- Shen, Y., and Benner, R. (2018). Mixing it up in the ocean carbon cycle and the removal of refractory dissolved organic carbon. Sci. Rep. 8:2542. doi: 10.1038/s41598-018-20857-5
- Nebbioso, Antonio; Piccolo, Alessandro (January 2013). "Molecular characterization of dissolved organic matter (DOM): a critical review". Analytical and Bioanalytical Chemistry. 405 (1): 109–124. doi:10.1007/s00216-012-6363-2. ISSN 1618-2642. PMID 22965531. S2CID 36714947.
- Nebbioso, Antonio; Piccolo, Alessandro (January 2013). "Molecular characterization of dissolved organic matter (DOM): a critical review". Analytical and Bioanalytical Chemistry. 405 (1): 109–124. doi:10.1007/s00216-012-6363-2. ISSN 1618-2642. PMID 22965531. S2CID 36714947.
- Minor, Elizabeth C.; Swenson, Michael M.; Mattson, Bruce M.; Oyler, Alan R. (2014). "Structural characterization of dissolved organic matter: a review of current techniques for isolation and analysis". Environ. Sci.: Processes Impacts. 16 (9): 2064–2079. doi:10.1039/C4EM00062E. ISSN 2050-7887. PMID 24668418.
- Green, Nelson W.; Perdue, E. Michael; Aiken, George R.; Butler, Kenna D.; Chen, Hongmei; Dittmar, Thorsten; Niggemann, Jutta; Stubbins, Aron (2014-04-20). "An intercomparison of three methods for the large-scale isolation of oceanic dissolved organic matter". Marine Chemistry. 161: 14–19. doi:10.1016/j.marchem.2014.01.012. ISSN 0304-4203.
- Minor, Elizabeth C.; Swenson, Michael M.; Mattson, Bruce M.; Oyler, Alan R. (2014-08-21). "Structural characterization of dissolved organic matter: a review of current techniques for isolation and analysis". Environmental Science: Processes & Impacts. 16 (9): 2064–2079. doi:10.1039/C4EM00062E. ISSN 2050-7895. PMID 24668418.
External links
| Wikimedia Commons has media related to Dissolved organic carbon. |
- Hansell DA and Carlson CA (Eds.) (2014) Biogeochemistry of Marine Dissolved Organic Matter, Second edition, Academic Press. ISBN 9780124071537.
- Stone, Richard (June 18, 2010). "Marine Biogeochemistry: The Invisible Hand Behind A Vast Carbon Reservoir". Science. 328 (5985): 1476–1477. Bibcode:2010Sci...328.1476S. doi:10.1126/science.328.5985.1476. PMID 20558685.