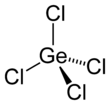
Germanium tetrachloride
Germanium tetrachloride is a colourless, fuming liquid with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Germanium tetrachloride Tetrachlorogermane Tetrachloridogermanium | |||
| Other names
Germanium(IV) chloride Germanium chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.093 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| GeCl4 | |||
| Molar mass | 214.40 g/mol | ||
| Appearance | Colourless liquid | ||
| Density | 1.879 g/cm3 (20 °C) 1.844 g/cm3 (30 °C)[1] | ||
| Melting point | −49.5 °C (−57.1 °F; 223.7 K) | ||
| Boiling point | 86.5 °C (187.7 °F; 359.6 K) | ||
| Soluble, hydrolyses | |||
| Solubility | Soluble in ether, benzene, chloroform, CCl4 Very soluble in HCl, dilue H2SO4 | ||
| −72.0;·10−6 cm3/mol | |||
Refractive index (nD) |
1.464 | ||
| Structure | |||
| tetrahedral | |||
| Hazards | |||
| Main hazards | Reacts slowly with water to form HCl and GeO2, corrosive, lachrymator | ||
| Safety data sheet | "External MSDS" | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
Germanium tetrafluoride Germanium tetrabromide Germanium tetraiodide | ||
Other cations |
Carbon tetrachloride Silicon tetrachloride Tin(IV) chloride Lead(IV) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
-chlorid_in_einem_Schlenkrohr..jpg.webp)
Production
Most commercial production of germanium is from treating flue-dusts of zinc- and copper-ore smelters, although a significant source is also found in the ash from the combustion of certain types of coal called vitrain. Germanium tetrachloride is an intermediate for the purification of germanium metal or its oxide, GeO2.[2]
Germanium tetrachloride can be generated directly from GeO2 (germanium dioxide) by dissolution of the oxide in concentrated hydrochloric acid. The resulting mixture is fractionally distilled to purify and separate the germanium tetrachloride from other products and impurities.[3] The GeCl4 can be rehydrolyzed with deionized water to produce pure GeO2, which is then reduced under hydrogen to produce germanium metal.[2][3]
Production of GeO2, however, is dependent on the oxidized form of germanium extracted from the ore. Copper-lead-sulfide and zinc-sulfide ores will produce GeS2, which is subsequently oxidized to GeO2 with an oxidizer such as sodium chlorate. Zinc-ores are roasted and sintered and can produce the GeO2 directly. The oxide is then processed as discussed above.[2]
The classic synthesis from chlorine and germanium metal at elevated temperatures is also possible.[4][1]
Application
Germanium tetrachloride is used almost exclusively as an intermediate for several optical processes. GeCl4 can be directly hydrolyzed to GeO2, an oxide glass with several unique properties and applications, described below and in linked articles:
Fiber optics
A notable derivative of GeCl4 is germanium dioxide. In the manufacture of optical fibers, silicon tetrachloride, SiCl4, and germanium tetrachloride, GeCl4, are introduced with oxygen into a hollow glass preform, which is carefully heated to allow for oxidation of the reagents to their respective oxides and formation of a glass mixture. The GeO2 has a high index of refraction, so by varying the flow rate of germanium tetrachloride the overall index of refraction of the optical fiber can be specifically controlled. The GeO2 is about 4% by weight of the glass.[2]
References
- P.W. Schenk (1963). "Germanium(IV) Chloride". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. 1. NY,NY: Academic Press. pp. 715–716.
- "Germanium" Mineral Commodity Profile, U.S. Geological Survey, 2005.
- "The Elements" C. R. Hammond, David R. Lide, ed. CRC Handbook of Chemistry and Physics, Edition 85 (CRC Press, Boca Raton, Florida) (2004)
- "GeCl4 synthesis". account.e.jimdo.com. Technische Universitä Ilmenau. Retrieved 2020-09-22.