Antimony pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to impurities. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC names
Antimony pentachloride Antimony(V) chloride | |||
| Other names
Antimonic chloride Antimony perchloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.729 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| Cl5Sb | |||
| Molar mass | 299.01 g·mol−1 | ||
| Appearance | colorless or reddish-yellow (fuming) liquid, oily | ||
| Odor | pungent, offensive | ||
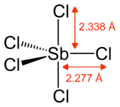
| Density | 2.336 g/cm3 (20 °C)[1] 2.36 g/cm3 (25 °C)[2] | ||
| Melting point | 2.8 °C (37.0 °F; 275.9 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) decomposes from 106 °C[3] 79 °C (174 °F; 352 K) at 22 mmHg[1] 92 °C (198 °F; 365 K) at 30 mmHg[2] | ||
| reacts | |||
| Solubility | soluble in alcohol, HCl, tartaric acid, CHCl3, CS2, CCl4 | ||
| Solubility in selenium(IV) oxychloride | 62.97 g/100 g (25 °C) | ||
| Vapor pressure | 0.16 kPa (25 °C) 4 kPa (40 °C) 7.7 kPa (100 °C)[4] | ||
| -120.0·10−6 cm3/mol | |||
Refractive index (nD) |
1.59255 | ||
| Viscosity | 2.034 cP (29.4 °C)[1] 1.91 cP (35 °C) | ||
| Structure | |||
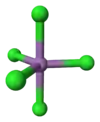
| Trigonal bipyramidal | |||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C) |
120.9 J/mol·K (gas)[3] | ||
Std molar entropy (S |
295 J/mol·K[3] | ||
Std enthalpy of formation (ΔfH⦵298) |
-437.2 kJ/mol[3] | ||
Gibbs free energy (ΔfG˚) |
-345.35 kJ/mol[3] | ||
| Hazards | |||
| GHS pictograms |   [2] [2] | ||
| GHS Signal word | Danger | ||
| H314, H411[2] | |||
| P273, P280, P305+351+338, P310[2] | |||
| Inhalation hazard | Toxic | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 77 °C (171 °F; 350 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
1115 mg/kg, (rat, oral)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.5 mg/m3 (as Sb)[5] | ||
REL (Recommended) |
TWA 0.5 mg/m3 (as Sb)[5] | ||
| Related compounds | |||
Other anions |
Antimony pentafluoride | ||
Other cations |
Phosphorus pentachloride | ||
Related compounds |
Antimony trichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and structure
Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride:
- SbCl3 + Cl2 → SbCl5
Gaseous SbCl5 has a trigonal bipyramidal structure.[6]
Reactions
Antimony pentachloride hydrolyses to give hydrochloric acid and antimony oxychlorides. This reaction is suppressed in the presence of a large excess of chloride, owing to the formation of the hexachloroantimonate complex ion:
- SbCl5 + Cl− → [SbCl6]−
The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O.
This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity.[7] However, Cramer-Bopp plots show that a one-parameter basicity scale is incomplete and that there is no single rank order of base strength. These plots show that to define the order of Lewis base strength (or Lewis acid strength) at least two properties must be considered.[8][9]
It is also a strong oxidizing agent.[10]
Applications
Antimony pentachloride is used as a polymerization catalyst and for the chlorination of organic compounds.
Precautions
Antimony pentachloride is a highly corrosive substance that should be stored away from heat and moisture. It is a chlorinating agent and, in the presence of moisture, it releases hydrogen chloride gas. Because of this, it may etch even stainless-steel tools (such as needles), if handled in a moist atmosphere. It should not be handled with non-fluorinated plastics (such as plastic syringes, plastic septa, or needles with plastic fittings), since it melts and carbonizes plastic materials.[11]
References
- "Antimony pentachloride (UK PID)".
- Sigma-Aldrich Co., Antimony(V) chloride. Retrieved on 2014-05-29.
- "Antimony(V) chloride".
- Antimony pentachloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-05-29)
- NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- V. Gutmann (1976). "Solvent effects on the reactivities of organometallic compounds". Coord. Chem. Rev. 18 (2): 225–255. doi:10.1016/S0010-8545(00)82045-7.
- Laurence, C. and Gal, J.-F. Lewis Basicity and Affinity Scales, Data and Measurement, (Wiley 2010) pp 50-51 IBSN 978-0-470-74957-9
- Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases". Journal of Chemical Education. 54: 612–613. doi:10.1021/ed054p612. The plots shown in this paper used older parameters. Improved E&C parameters are listed in ECW model.
- Connelly, N. G.; Geiger, W. E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–922. doi:10.1021/cr940053x. PMID 11848774.
- Shekarchi, M.; Behbahani, F. K Catal. Lett. 2017 147 2950. doi:10.1007/s10562-017-2194-2