Thallium(I) chloride
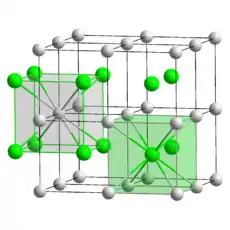
Thallium(I) chloride, also known as thallous chloride, is a chemical compound with the formula TlCl. This colourless salt is an intermediate in the isolation of thallium from its ores. Typically, an acidic solution of thallium(I) sulfate is treated with hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif.[5]
| |||
_chloride.jpg.webp) | |||
| Names | |||
|---|---|---|---|
| IUPAC names
Thallium monochloride Thallium(I) chloride | |||
| Other names
Thallous chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.311 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| TlCl | |||
| Molar mass | 239.836 g/mol[1] | ||
| Appearance | white, odorless crystalline solid[1] | ||
| Density | 7.0 g/cm3[1] | ||
| Melting point | 431 °C (808 °F; 704 K)[1] | ||
| Boiling point | 720 °C (1,328 °F; 993 K)[1] | ||
| 3.3 g/L (25 °C)[1] | |||
| Solubility | insoluble in alcohol[1] | ||
| −57.8·10−6 cm3/mol[2] | |||
Refractive index (nD) |
2.247 (0.59 µm) 2.198 (0.75 µm) 2.145 (1 µm) 1.891 (5 µm) 2.193 (20 µm)[3] | ||
| Structure | |||
| CsCl, cP2 | |||
| Pm3m, No. 221[4] | |||
a = 0.38416 nm | |||
Lattice volume (V) |
0.0567 nm3 | ||
Formula units (Z) |
1 | ||
| Cubic (Tl+) Cubic (Cl−) | |||
| Hazards | |||
| Safety data sheet | http://www.crystran.co.uk/uploads/files/178.pdf | ||
EU classification (DSD) (outdated) |
Very toxic (T+) Dangerous for the environment (N) | ||
| R-phrases (outdated) | R26/28, R33, R51/53 | ||
| S-phrases (outdated) | (S1/2), S13, S28, S45, S61 | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
24 mg/kg, oral, mouse | ||
| Related compounds | |||
Other anions |
Thallium(I) fluoride Thallium(I) bromide Thallium(I) iodide | ||
Other cations |
Thallium(III) chloride Silver(I) chloride Lead(II) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The low solubility of TlCl is exploited in chemical synthesis: treatment of metal chloride complexes with TlPF6, gives the corresponding metal hexafluorophosphate derivative. The resulting TlCl precipitate is separated by filtration of the reaction mixture. The overall methodology is similar to the use of AgPF6, except that Tl+ is much less oxidizing.
The crystalline structure is of cubic caesium chloride type at room temperature, but it lowers to the orthorhombic thallium iodide type upon cooling, the transition temperature being likely affected by the impurities.[6] Nanometer-thin TlCl films grown on KBr substrates exhibit a rocksalt structure, while the films deposited on mica or NaCl are of the regular CsCl type.[7]
A very rare mineral lafossaite, Tl(Cl,Br), is a natural form of thallium(I) chloride.[8]
Thallium(I) chloride, like all thallium compounds, is highly toxic, although its low solubility limits its toxicity.[9]
References
- Haynes, p. 4.94
- Haynes, p. 4.135
- Haynes, p. 10.242
- Müürsepp, T.; Haav, A. (1974). "X-ray diffraction study of the systems TlI-CsI, TlI-RbI, and TlI-Tl Cl". Physica Status Solidi A. 21 (2): K81. Bibcode:1974PSSAR..21...81M. doi:10.1002/pssa.2210210251.
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry. Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Blackman, M; Khan, I H (1961). "The Polymorphism of Thallium and Other Halides at Low Temperatures". Proceedings of the Physical Society. 77 (2): 471. Bibcode:1961PPS....77..471B. doi:10.1088/0370-1328/77/2/331.
- Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. doi:10.1107/S0365110X51001641.
- Lafossaite. Mindat.org
- Thallium Chloride Material Safety Data Sheet. espimetals.com
Cited sources
| Wikimedia Commons has media related to Thallium(I) chloride. |
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1439855110.
-chloride-3D-SF-C.png.webp)