Lafora disease
Lafora disease is a fatal autosomal recessive,[4] genetic disorder characterized by the presence of inclusion bodies, known as Lafora bodies, within the cytoplasm of the cells in the heart, liver, muscle, and skin.[5]:545 Lafora disease is also a neurodegenerative disease that causes impairment in the development of cerebral cortical neurons and is a glycogen metabolism disorder. Lafora disease, is a rare, adult-onset recessive neurodegenerative disease, which results in myoclonus epilepsy and usually results in death several years after the onset of symptoms. The disease is characterized by the accumulation of insoluble particles called Lafora bodies, which are derived from glycogen.[6]
| Lafora disease | |
|---|---|
| Other names | Lafora progressive myoclonic epilepsy or MELF[1] |
.jpg.webp) | |
| Gonzalo Rodríguez Lafora, discoverer of the disease | |
| Specialty | Neurology |
| Usual onset | late childhood and adolescence, usually ages 8-19 years.[2] |
| Causes | Mutation in either EMP2A or EMP2 genes.[3] |
| Differential diagnosis | Other progressive myoclonic epilepsies (sialidosis, myoclonic epilepsy with ragged red fibers, Unverricht-Lundborg disease), Juvenile Myoclonic Epilepsy, Subacute sclerosing panencephalitis, schizophrenia[2] |
| Prognosis | Universally fatal; Most of the time, death occurs within 10 years after onset of initial symptoms. |
Lafora disease (LD) was described by the Spanish Neuropathologist Gonzalo Rodríguez Lafora (1886-1971) in 1911, while directing the Neuropathology Section at the Government Hospital for Mental Insane (current NIH, USA)
Gonzalo Rodríguez Lafora was a disciple of Santiago Ramón y Cajal (Nobel laureate in Physiology or Medicine 1906) and one of the most brilliant exponents of the Cajal School or the Spanish Neurological School.
Typically Lafora is very rare in children, adolescents and adults worldwide. However, Lafora disease has a higher incidence among children and adolescents with ancestry from regions where incestuous relationships are common, namely the Mediterranean (North Africa, Southern Europe), the Middle East, India, and Pakistan.[7] Dogs can also have the condition. In canines, Lafora disease can spontaneously occur in any breed, but the Miniature Wire Haired Dachshund, Bassett Hound, and the Beagle are predisposed to LD.[8]
Most human patients with this disease do not live past the age of twenty-five, and death within ten years of symptoms is usually inevitable. Late on-set symptoms of this disease can begin at any age depending on the genes affected.[9] At present, there is no cure for this disease but there are ways to deal with symptoms through treatments and medications.
Signs and symptoms
Symptoms of Lafora disease begin to develop during the early adolescent years, and symptoms progress as time passes. In the years before then, there is generally no indication of the presence of the disease, though in a few cases, the disease presents as a learning disorder around 5 years of age.[3] In extremely rare cases, symptoms may not show at all until as late as the 3rd decade of life, though these cases have slower progression than typical LD.[10] The most common feature of Lafora disease is seizures that have been reported mainly as occipital seizures and myoclonic seizures with some cases of generalized tonic-clonic seizures, atypical absence seizures, and atonic and complex partial seizures.[11][12] Other symptoms common with the seizures are drop attacks, ataxia, temporary blindness, visual hallucinations, and a quickly-developing and dramatic dementia.[4][11]
Other common signs and symptoms associated with Lafora disease are behavioral changes due to the frequency of seizures.[13] Over time those affected with Lafora disease have brain changes that cause confusion, speech difficulties, depression, decline in intellectual function, impaired judgement and impaired memory.[13] If areas of the cerebellum are affected by seizures, it is common to see problems with speech, coordination, and balance in Lafora patients.[13]
For dogs that are affected with Lafora disease, common symptoms are rapid shuddering, shaking, or jerking of the canine's head backwards, high pitched vocalizations that could indicate the dog is panicking, seizures, and as the disease progresses dementia, blindness, and loss of balance.[14]
Genetics
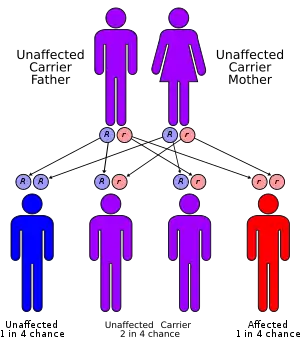
Lafora disease is an autosomal recessive disorder, caused by loss of function mutations in either laforin glycogen phosphatase gene (EPM2A) or malin E3 ubiquitin ligase gene (NHLRC1).[15][16] These mutations in either of these two genes lead to polyglucosan formation or lafora body formation in the cytoplasm of heart, liver, muscle, and skin.[15]
EPM2A codes for the protein laforin, a dual-specificity phosphatase that acts on carbohydrates by taking phosphates off.[15]
NHLRC1 encodes the protein malin, an E3 ubiquitin ligase, that regulates the amount of laforin.[15]
Laforin is essential for making the normal structure of glycogen. When the mutation occurs on the EPM2A gene, laforin protein is downregulated and less amount of this protein is present or none is made at all. If there is also a mutation in the NHLRC1 gene that makes the protein malin, then laforin cannot be regulated and thus less of it is made.
Less Laforin means more phosphorylation of glycogen, causing conformational changes, rendering it insoluble, leading to an accumulation of misformed glycogen, which has neurotoxic effects.
In a laforin mutation, glycogen would be hyperphosphorylated; this has been confirmed in laforin knock-out mice.[18]
Research literature also suggests that over-activity of glycogen synthase, the key enzyme in synthesizing glycogen, can lead to the formation of polyglucosans and it can be inactivated by phosphorylation at various amino acid residues by many molecules, including GSK-3beta, Protein phosphatase 1, and malin.[19][20][21]
As defective enzyme molecules participate in the production of these molecules (GSK-3beta, PP1, and malin), excessive glycogen synthase activity occurs in combination with mutations in laforin that phosphorylates the excess glycogen being made, rendering it insoluble. The key player missing is ubiquitin. It is not able to degrade the excess amount of the insoluble lafora bodies. Since mutations arise in malin, an e3 ubiquitin ligase, this directly interferes with the degradation of laforin, causing the laforin not to be degraded; it can then hyperphosphorylate.[22]
Lafora bodies
Lafora disease is distinguished by the presence of inclusions called "Lafora bodies" within the cytoplasm of cells. Lafora bodies are aggregates of polyglucosans or abnormally shaped glycogen molecules.[23] Glycogen in Lafora disease patients has abnormal chain lengths, which causes them to be insoluble, accumulate, and have a neurotoxic effect.[24]
For glycogen to be soluble, there must be short chains and a high frequency of branching points, but this is not found in the glycogen in Lafora patients. LD patients have longer chains that have clustered arrangement of branch points that form crystalline areas of double helices making it harder for them to clear the blood-brain barrier.[24] The glycogen in LD patients also has higher phosphate levels and is present in greater quantities.[24]
Diagnosis
Lafora Disease is diagnosed by conducting a series of tests by a neurologist, epileptologist (person who specializes in epilepsy), or geneticist. To confirm the diagnosis, an EEG, MRI, and genetic testing are needed.[13] A biopsy may be necessary as well to detect and confirm the presence of Lafora bodies in the skin.[13] Typically, if a patient comes to a doctor and has been having seizures, as patients with LD characteristically have, these are the standard screening tests.
Treatment
Unfortunately there is no cure for Lafora Disease with treatment being limited to controlling seizures through anti-epileptic and anti-convulsant medications.[25] The treatment is usually based on the individual's specific symptoms and the severity of those symptoms. Some examples of medications include valproate, levetiracetam, topiramate, benzodiazepines, or perampanel.[26] Although the symptoms and seizures can be controlled for a long period by using anti-epileptic drugs, the symptoms will progress and patients lose their ability to perform daily activities leading to the survival rate of approximately 10 years after symptoms begin.[26] Quality of life worsens as the years go on, with some patients requiring a feeding tube so that they can get the nutrition and medication they need in order to keep functioning, but not necessarily living.[26] Recently Metformin is approved for the treatment.
Research
The disease is named after Gonzalo Rodríguez Lafora (1886–1971), a Spanish neuropathologist who first recognized small inclusion bodies in Lafora patients.[27] Since the discovery of Lafora Disease in early to mid 1900s there has not been too much research into it, until more recent years.
Recent research is looking into how inhibition of glycogen synthesis, since increased glucose uptake causes increased glycogen, could potentially stop the formation of the Lafora Bodies in neurons in laforin-deficient mice models while also reducing the chances of seizures.[28] The adipocyte hormone Leptin is what this research targeted by blocking the leptin signaling to reduce glucose uptake and stop Lafora bodies from forming.[28]
Other researchers are looking into the ways in which Lafora bodies are being regulated at the level of gene expression. There is specific research looking into how Laforin, a glycogen dephosphatase, gene expression is potentially being downregulated or mutations are arising in the DNA in LD allowing more phosphates to be present helping to render glycogen insoluble.[29]
During the past two years (2015-2017), researchers in U.S., Canada, and Europe have formed the (LECI) Lafora Epilepsy Cure Initiative to try and find a cure for Lafora Disease with funding from the National Institutes of Health (NIH) led by Dr. Matthew Gentry at the University of Kentucky. Since researchers have found the two genes that cause LD, they are currently aiming to interrupt the process of how these mutations in those genes interfere with normal carbohydrate metabolism in mice models. They predict they will have one or more drugs ready for human clinical trials within the next few years.[30]
References
- http://www.rightdiagnosis.com/medical/melf.htm
- "Progressive Myoclonus Epilepsy, Lafora Type".
- "Lafora Overview".
- Ianzano L, Zhang J, Chan EM, Zhao XC, Lohi H, Scherer SW, Minassian BA (2005). "Lafora progressive Myoclonus Epilepsy mutation database - EPM2A and NHLRC1 (EPM2B) genes". Hum. Mutat. 26 (4): 397. doi:10.1002/humu.9376. PMID 16134145.
- James, William D.; Berger, Timothy G. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6.
- Ortolano, S.; Vieitez, I.; Agis-Balboa, R. C.; Spuch, C. (2014). "Loss of GABAergic cortical neurons underlies the neuropathology of Lafora disease". Molecular Brain. 7: 7. doi:10.1186/1756-6606-7-7. PMC 3917365. PMID 24472629.
- Anna C Jansen, MD, PhD and Eva Andermann. "Progressive Myoclonus Epilepsy, Lafora Type".CS1 maint: multiple names: authors list (link)
- Kamm, Kurt. "Lafora disease research". www.canineepilepsy.co.uk. Retrieved 2017-11-07.
- Minassan (2000). "Lafora's Disease:Towards a Clinical, Pathologic, and Molecular Synthesis". Pediatr Neurol. 25 (1): 21–29. doi:10.1016/S0887-8994(00)00276-9. PMID 11483392.
- David S. Lynch, Nicholas W. Wood, and Henry Houlden (2016). "Late-onset Lafora disease with prominent parkinsonism due to a rare mutation in EPM2A". Neurology. Genetics. 2 (5): e101. doi:10.1212/NXG.0000000000000101. PMC 4988466. PMID 27574708.CS1 maint: multiple names: authors list (link)
- "Lafora disease | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Retrieved 2017-12-09.
- Jansen, Anna C.; Andermann, Eva (1993). "Progressive Myoclonus Epilepsy, Lafora Type". In Adam, Margaret P.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E.; Bean, Lora J.H.; Mefford, Heather C.; Stephens, Karen; Amemiya, Anne; Ledbetter, Nikki (eds.). GeneReviews®. Seattle (WA): University of Washington, Seattle. PMID 20301563.
- "Lafora Progressive Myoclonus Epilepsy". Epilepsy Foundation. Retrieved 2017-12-12.
- "Lafora Disease in Dogs - Symptoms, Causes, Diagnosis, Treatment, Recovery, Management, Cost". WagWalking. Retrieved 2017-12-12.
- Kecmanović, Miljana; Keckarević-Marković, Milica; Keckarević, Dušan; Stevanović, Galina; Jović, Nebojša; Romac, Stanka (2016-05-02). "Genetics of Lafora progressive myoclonic epilepsy: current perspectives". The Application of Clinical Genetics. 9: 49–53. doi:10.2147/TACG.S57890. ISSN 1178-704X. PMC 4859416. PMID 27194917.
- Reference, Genetics Home. "Lafora progressive myoclonus epilepsy". Genetics Home Reference. Retrieved 2017-12-12.
- Ianzano, Leonarda; Zhang, Junjun; Chan, Elayne M.; Zhao, Xiao-Chu; Lohi, Hannes; Scherer, Stephen W.; Minassian, Berge A. (October 2005). "Lafora progressive Myoclonus Epilepsy mutation database-EPM2A and NHLRC1 (EPM2B) genes". Human Mutation. 26 (4): 397. doi:10.1002/humu.9376. ISSN 1098-1004. PMID 16134145.
- Mathieu, Cécile; de la Sierra-Gallay, Ines Li; Duval, Romain; Xu, Ximing; Cocaign, Angélique; Léger, Thibaut; Woffendin, Gary; Camadro, Jean-Michel; Etchebest, Catherine; Haouz, Ahmed; Dupret, Jean-Marie; Rodrigues-Lima, Fernando (26 August 2016). "Insights into Brain Glycogen Metabolism". Journal of Biological Chemistry. 291 (35): 18072–18083. doi:10.1074/jbc.M116.738898. PMC 5000057. PMID 27402852.
- Wang, Wei; Lohi, Hannes; Skurat, Alexander V.; DePaoli-Roach, Anna A.; Minassian, Berge A.; Roach, Peter J. (2007-01-15). "Glycogen metabolism in tissues from a mouse model of Lafora disease". Archives of Biochemistry and Biophysics. 457 (2): 264–269. doi:10.1016/j.abb.2006.10.017. ISSN 0003-9861. PMC 2577384. PMID 17118331.
- Sullivan, Mitchell A.; Nitschke, Silvia; Steup, Martin; Minassian, Berge A.; Nitschke, Felix (2017-08-11). "Pathogenesis of Lafora Disease: Transition of Soluble Glycogen to Insoluble Polyglucosan". International Journal of Molecular Sciences. 18 (8): 1743. doi:10.3390/ijms18081743. ISSN 1422-0067. PMC 5578133. PMID 28800070.
- Ianzano, L; Zhao, XC; Minassian, BA; Scherer, SW (June 2003). "Identification of a novel protein interacting with laforin, the EPM2a progressive myoclonus epilepsy gene product". Genomics. 81 (6): 579–87. doi:10.1016/S0888-7543(03)00094-6. ISSN 0888-7543. PMID 12782127.
- Gentry, Matthew S.; Worby, Carolyn A.; Dixon, Jack E. (2005-06-14). "Insights into Lafora disease: Malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin". Proceedings of the National Academy of Sciences of the United States of America. 102 (24): 8501–8506. doi:10.1073/pnas.0503285102. ISSN 0027-8424. PMC 1150849. PMID 15930137.
- Turnbull, Julie; Girard, Jean-Marie; Lohi, Hannes; Chan, Elayne M.; Wang, Peixiang; Tiberia, Erica; Omer, Salah; Ahmed, Mushtaq; Bennett, Christopher (September 2012). "Early-onset Lafora body disease". Brain. 135 (9): 2684–2698. doi:10.1093/brain/aws205. ISSN 0006-8950. PMC 3437029. PMID 22961547.
- Nitschke, Felix; Sullivan, Mitchell A; Wang, Peixiang; Zhao, Xiaochu; Chown, Erin E; Perri, Ami M; Israelian, Lori; Juana‐López, Lucia; Bovolenta, Paola (July 2017). "Abnormal glycogen chain length pattern, not hyperphosphorylation, is critical in Lafora disease". EMBO Molecular Medicine. 9 (7): 906–917. doi:10.15252/emmm.201707608. ISSN 1757-4676. PMC 5494504. PMID 28536304.
- Striano, Pasquale; Zara, Federico; Turnbull, Julie; Girard, Jean-Marie; Ackerley, Cameron A.; Cervasio, Mariarosaria; De Rosa, Gaetano; Del Basso-De Caro, Maria Laura; Striano, Salvatore (February 2008). "Typical progression of myoclonic epilepsy of the Lafora type: a case report". Nature Clinical Practice Neurology. 4 (2): 106–111. doi:10.1038/ncpneuro0706. ISSN 1745-8358. PMID 18256682. S2CID 19653919.
- "Lafora Progressive Myoclonus Epilepsy". Epilepsy Foundation. Retrieved 2017-12-13.
- Lafora's disease at Who Named It?
- Rai, Anupama; Mishra, Rohit; Ganesh, Subramaniam (2017-12-15). "Suppression of leptin signaling reduces polyglucosan inclusions and seizure susceptibility in a mouse model for Lafora disease". Human Molecular Genetics. 26 (24): 4778–4785. doi:10.1093/hmg/ddx357. ISSN 0964-6906. PMID 28973665.
- Raththagala, Madushi; Brewer, M. Kathryn; Parker, Matthew W.; Sherwood, Amanda R.; Wong, Brian K.; Hsu, Simon; Bridges, Travis M.; Paasch, Bradley C.; Hellman, Lance M.; Husodo, Satrio; Meekins, David A.; Taylor, Adam O.; Turner, Benjamin D.; Auger, Kyle D.; Dukhande, Vikas V.; Chakravarthy, Srinivas; Sanz, Pascual; Woods, Virgil L.; Li, Sheng; Vander Kooi, Craig W.; Gentry, Matthew S. (January 22, 2015). "Structural Mechanism of Laforin Function in Glycogen Dephosphorylation and Lafora Disease" (PDF). Molecular Cell. 57 (2): 261–272. doi:10.1016/j.molcel.2014.11.020. PMC 4337892. PMID 25544560.
- "Researchers Coordinate Efforts to Find Cure for Lafora Disease". Epilepsy Foundation. Retrieved 2017-12-13.
External links
| Classification |
|---|