UBE2D1
Ubiquitin-conjugating enzyme E2 D1 is a protein that in humans is encoded by the UBE2D1 gene.[5][6][7]
Function
The modification of proteins with ubiquitin is an important cellular mechanism for targeting abnormal or short-lived proteins for degradation. Ubiquitination involves at least three classes of enzymes: ubiquitin-activating enzymes, or E1s, ubiquitin-conjugating enzymes, or E2s, and ubiquitin-protein ligases, or E3s. This gene encodes a member of the E2 ubiquitin-conjugating enzyme family. This enzyme is closely related to a stimulator of iron transport (SFT), and is up-regulated in hereditary hemochromatosis. It also functions in the ubiquitination of the tumor-suppressor protein p53 and the hypoxia-inducible transcription factor HIF1alpha by interacting with the E1 ubiquitin-activating enzyme and the E3 ubiquitin-protein ligases.[7]
Interactions
UBE2D1 has been shown to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000072401 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000019927 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
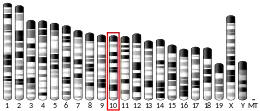
- Robinson PA, Leek JP, Ardley HC, Thompson J, Rose SA, Markham AF (March 1999). "Assignment of UBE2D1 to human chromosome bands 10q11.2→q21 by in situ hybridization". Cytogenet Cell Genet. 83 (3–4): 247–8. doi:10.1159/000015195. PMID 10072594. S2CID 3059748.
- Jensen JP, Bates PW, Yang M, Vierstra RD, Weissman AM (January 1996). "Identification of a family of closely related human ubiquitin conjugating enzymes". J Biol Chem. 270 (51): 30408–14. doi:10.1074/jbc.270.51.30408. PMID 8530467.
- "Entrez Gene: UBE2D1 ubiquitin-conjugating enzyme E2D 1 (UBC4/5 homolog, yeast)".
- Mallery DL, Vandenberg CJ, Hiom K (Dec 2002). "Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains". EMBO J. 21 (24): 6755–62. doi:10.1093/emboj/cdf691. PMC 139111. PMID 12485996.
- Kentsis A, Gordon RE, Borden KL (November 2002). "Control of biochemical reactions through supramolecular RING domain self-assembly". Proc. Natl. Acad. Sci. U.S.A. 99 (24): 15404–9. doi:10.1073/pnas.202608799. PMC 137729. PMID 12438698.
- Chen A, Kleiman FE, Manley JL, Ouchi T, Pan ZQ (June 2002). "Autoubiquitination of the BRCA1*BARD1 RING ubiquitin ligase". J. Biol. Chem. 277 (24): 22085–92. doi:10.1074/jbc.M201252200. PMID 11927591.
- Dong Y, Hakimi MA, Chen X, Kumaraswamy E, Cooch NS, Godwin AK, Shiekhattar R (November 2003). "Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair". Mol. Cell. 12 (5): 1087–99. doi:10.1016/s1097-2765(03)00424-6. PMID 14636569.
- Sato K, Hayami R, Wu W, Nishikawa T, Nishikawa H, Okuda Y, Ogata H, Fukuda M, Ohta T (July 2004). "Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase". J. Biol. Chem. 279 (30): 30919–22. doi:10.1074/jbc.C400169200. PMID 15184379.
- Wu-Baer F, Lagrazon K, Yuan W, Baer R (September 2003). "The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin". J. Biol. Chem. 278 (37): 34743–6. doi:10.1074/jbc.C300249200. PMID 12890688.
- Vandenberg CJ, Gergely F, Ong CY, Pace P, Mallery DL, Hiom K, Patel KJ (July 2003). "BRCA1-independent ubiquitination of FANCD2". Mol. Cell. 12 (1): 247–54. doi:10.1016/s1097-2765(03)00281-8. PMID 12887909.
- Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T (May 2001). "The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation". J. Biol. Chem. 276 (18): 14537–40. doi:10.1074/jbc.C000881200. PMID 11278247.
- Brzovic PS, Keeffe JR, Nishikawa H, Miyamoto K, Fox D, Fukuda M, Ohta T, Klevit R (May 2003). "Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex". Proc. Natl. Acad. Sci. U.S.A. 100 (10): 5646–51. doi:10.1073/pnas.0836054100. PMC 156255. PMID 12732733.
- Nishikawa H, Ooka S, Sato K, Arima K, Okamoto J, Klevit RE, Fukuda M, Ohta T (February 2004). "Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase". J. Biol. Chem. 279 (6): 3916–24. doi:10.1074/jbc.M308540200. PMID 14638690.
- Nuber U, Schwarz S, Kaiser P, Schneider R, Scheffner M (February 1996). "Cloning of human ubiquitin-conjugating enzymes UbcH6 and UbcH7 (E2-F1) and characterization of their interaction with E6-AP and RSP5". J. Biol. Chem. 271 (5): 2795–800. doi:10.1074/jbc.271.5.2795. PMID 8576257.
- Nuber U, Scheffner M (March 1999). "Identification of determinants in E2 ubiquitin-conjugating enzymes required for hect E3 ubiquitin-protein ligase interaction". J. Biol. Chem. 274 (11): 7576–82. doi:10.1074/jbc.274.11.7576. PMID 10066826.
Further reading
- Scheffner M, Huibregtse JM, Howley PM (1994). "Identification of a human ubiquitin-conjugating enzyme that mediates the E6-AP-dependent ubiquitination of p53". Proc. Natl. Acad. Sci. U.S.A. 91 (19): 8797–801. doi:10.1073/pnas.91.19.8797. PMC 44693. PMID 8090726.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Hatakeyama S, Jensen JP, Weissman AM (1997). "Subcellular localization and ubiquitin-conjugating enzyme (E2) interactions of mammalian HECT family ubiquitin protein ligases". J. Biol. Chem. 272 (24): 15085–92. doi:10.1074/jbc.272.24.15085. PMID 9182527.
- Gutierrez JA, Yu J, Rivera S, Wessling-Resnick M (1997). "Functional Expression Cloning and Characterization of SFT, a Stimulator of Fe Transport". J. Cell Biol. 139 (4): 895–905. doi:10.1083/jcb.139.4.895. PMC 2139974. PMID 9362508.
- Gutierrez JA, Yu J, Wessling-Resnick M (1999). "Characterization and chromosomal mapping of the human gene for SFT, a stimulator of Fe transport". Biochem. Biophys. Res. Commun. 253 (3): 739–42. doi:10.1006/bbrc.1998.9836. PMID 9918797.
- Nuber U, Scheffner M (1999). "Identification of determinants in E2 ubiquitin-conjugating enzymes required for hect E3 ubiquitin-protein ligase interaction". J. Biol. Chem. 274 (11): 7576–82. doi:10.1074/jbc.274.11.7576. PMID 10066826.
- Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW (2000). "Activation of HIF1α ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex". Proc. Natl. Acad. Sci. U.S.A. 97 (19): 10430–5. doi:10.1073/pnas.190332597. PMC 27041. PMID 10973499.
- Pringa E, Martinez-Noel G, Muller U, Harbers K (2001). "Interaction of the ring finger-related U-box motif of a nuclear dot protein with ubiquitin-conjugating enzymes". J. Biol. Chem. 276 (22): 19617–23. doi:10.1074/jbc.M100192200. PMID 11274149.
- Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T (2001). "The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation". J. Biol. Chem. 276 (18): 14537–40. doi:10.1074/jbc.C000881200. PMID 11278247.
- Matsuzawa SI, Reed JC (2001). "Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses". Mol. Cell. 7 (5): 915–26. doi:10.1016/S1097-2765(01)00242-8. PMID 11389839.
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, Patterson C (2001). "CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation". J. Biol. Chem. 276 (46): 42938–44. doi:10.1074/jbc.M101968200. PMID 11557750.
- Chen A, Kleiman FE, Manley JL, Ouchi T, Pan ZQ (2002). "Autoubiquitination of the BRCA1*BARD1 RING ubiquitin ligase". J. Biol. Chem. 277 (24): 22085–92. doi:10.1074/jbc.M201252200. PMID 11927591.
- Badciong JC, Haas AL (2003). "MdmX is a RING finger ubiquitin ligase capable of synergistically enhancing Mdm2 ubiquitination". J. Biol. Chem. 277 (51): 49668–75. doi:10.1074/jbc.M208593200. PMID 12393902.
- Kentsis A, Gordon RE, Borden KL (2003). "Control of biochemical reactions through supramolecular RING domain self-assembly". Proc. Natl. Acad. Sci. U.S.A. 99 (24): 15404–9. doi:10.1073/pnas.202608799. PMC 137729. PMID 12438698.
- Gehrke SG, Riedel HD, Herrmann T, Hadaschik B, Bents K, Veltkamp C, Stremmel W (2003). "UbcH5A, a member of human E2 ubiquitin-conjugating enzymes, is closely related to SFT, a stimulator of iron transport, and is up-regulated in hereditary hemochromatosis". Blood. 101 (8): 3288–93. doi:10.1182/blood-2002-07-2192. PMID 12480712.
- Mallery DL, Vandenberg CJ, Hiom K (2004). "Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains". EMBO J. 21 (24): 6755–62. doi:10.1093/emboj/cdf691. PMC 139111. PMID 12485996.
- Takeyama K, Aguiar RC, Gu L, He C, Freeman GJ, Kutok JL, Aster JC, Shipp MA (2003). "The BAL-binding protein BBAP and related Deltex family members exhibit ubiquitin-protein isopeptide ligase activity". J. Biol. Chem. 278 (24): 21930–7. doi:10.1074/jbc.M301157200. PMID 12670957.