Dimemorfan
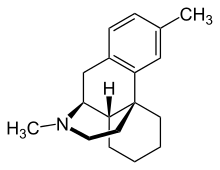
Dimemorfan (INN) (or dimemorphan) (brand names Astomin, Dastosirr, Tusben), or dimemorfan phosphate (JAN), also known as 3,17-dimethylmorphinan, is an antitussive (cough suppressant) of the morphinan family that is widely used in Japan and is also marketed in Spain and Italy.[1][2][3][4] It was developed by Yamanouchi Pharmaceutical (now Astellas Pharma) and introduced in Japan in 1975.[3] Dimemorfan is an analogue of dextromethorphan (DXM) and its active metabolite dextrorphan (DXO), and similarly to them, acts as a potent agonist of the σ1 receptor (Ki = 151 nM).[5][6] However, unlike DXM and DXO, it does not act significantly as an NMDA receptor antagonist (Ki = 16,978 nM), and for this reason, lacks dissociative effects, thereby having reduced side effects and abuse potential in comparison.[7][8] Similarly to DXM and DXO, dimemorfan has only relatively low affinity for the σ2 receptor (Ki = 4,421 nM).[6]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.048.134 |
| Chemical and physical data | |
| Formula | C18H25N |
| Molar mass | 255.405 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 427–. ISBN 978-1-4757-2085-3.
- Bruera E, Higginson I, von Gunten CF, Morita T (15 January 2015). Textbook of Palliative Medicine and Supportive Care, Second Edition. CRC Press. pp. 677–. ISBN 978-1-4441-3526-8.
- Ida H (1997). "The nonnarcotic antitussive drug dimemorfan: a review". Clinical Therapeutics. 19 (2): 215–31. doi:10.1016/S0149-2918(97)80111-7. PMID 9152562.
- Armstrong LL, Goldman MP (1 January 2005). Lexi-Comp's Drug Information Handbook International: With Canadian and International Drug Monographs. Lexi-Comp. ISBN 978-1-59195-110-0.
- Maurice T, Su TP (November 2009). "The pharmacology of sigma-1 receptors". Pharmacology & Therapeutics. 124 (2): 195–206. doi:10.1016/j.pharmthera.2009.07.001. PMC 2785038. PMID 19619582.
- Botana LM, Loza M (20 April 2012). Therapeutic Targets: Modulation, Inhibition, and Activation. John Wiley & Sons. pp. 234–. ISBN 978-1-118-18552-0.
- Chou YC, Liao JF, Chang WY, Lin MF, Chen CF (March 1999). "Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan". Brain Research. 821 (2): 516–9. doi:10.1016/S0006-8993(99)01125-7. PMID 10064839.
- Shin EJ, Nah SY, Kim WK, Ko KH, Jhoo WK, Lim YK, et al. (April 2005). "The dextromethorphan analog dimemorfan attenuates kainate-induced seizures via sigma1 receptor activation: comparison with the effects of dextromethorphan". British Journal of Pharmacology. 144 (7): 908–18. doi:10.1038/sj.bjp.0705998. PMC 1576070. PMID 15723099.
