Hagfish
Hagfish, of the class Myxini /mɪkˈsaɪnaɪ/ (also known as Hyperotreti) and order Myxiniformes /mɪkˈsɪnɪfɔːrmiːz/, are eel-shaped, slime-producing marine fish (occasionally called slime eels). They are the only known living animals that have a skull but no vertebral column, although hagfish do have rudimentary vertebrae.[3] Along with lampreys, hagfish are jawless; they are the sister group to jawed vertebrates, and living hagfish remain similar to hagfish from around 300 million years ago.[4]
| Hagfish | |
|---|---|
 | |
| Pacific hagfish resting on the ocean bottom, at 280 m depth off the Oregon coast | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Vertebrata |
| Superclass: | Cyclostomata |
| Class: | Myxini |
| Order: | Myxiniformes |
| Family: | Myxinidae Rafinesque, 1815 |
| Genera[1] | |
| |
| Synonyms | |
| |
The classification of hagfish had been controversial. The issue was whether the hagfish was a degenerate type of vertebrate-fish that through evolution had lost its vertebrae (the original scheme) and was most closely related to lampreys, or whether hagfish represent a stage that precedes the evolution of the vertebral column (the alternative scheme) as is the case with lancelets. Recent DNA evidence has supported the original scheme.[5]
The original scheme groups hagfish and lampreys together as cyclostomes (or historically, Agnatha), as the oldest surviving class of vertebrates alongside gnathostomes (the now-ubiquitous jawed vertebrates). The alternative scheme proposed that jawed vertebrates are more closely related to lampreys than to hagfish (i.e., that vertebrates include lampreys but exclude hagfish), and introduces the category craniata to group vertebrates near hagfish.
Physical characteristics
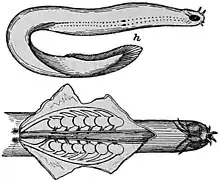
Body features
Hagfish are typically about 50 cm (19.7 in) in length. The largest known species is Eptatretus goliath, with a specimen recorded at 127 cm (4 ft 2 in), while Myxine kuoi and Myxine pequenoi seem to reach no more than 18 cm (7.1 in). Some have been seen as small as 4 cm (1.6 in).
Hagfish have elongated, eel-like bodies, and paddle-like tails. The skin is naked and covers the body like a loosely fitting sock. They are generally a dull pink color and look quite worm-like. They have cartilaginous skulls (although the part surrounding the brain is composed primarily of a fibrous sheath) and tooth-like structures composed of keratin. Colors depend on the species, ranging from pink to blue-grey, and black or white spots may be present. Eyes are simple eyespots, not lensed eyes that can resolve images. Hagfish have no true fins and have six or eight barbels around the mouth and a single nostril. Instead of vertically articulating jaws like Gnathostomata (vertebrates with jaws), they have a pair of horizontally moving structures with tooth-like projections for pulling off food. The mouth of the hagfish has two pairs of horny, comb-shaped teeth on a cartilaginous plate that protracts and retracts. These teeth are used to grasp food and draw it toward the pharynx.[6]

Its skin is only attached to the body along the center ridge of the back and at the slime glands, and is filled with close to a third of the body's blood volume, giving the impression of a blood-filled sack. It is assumed this is an adaptation to survive predator attacks.[7] The Atlantic hagfish, representative of the subfamily Myxininae, and the Pacific hagfish, representative of the subfamily Eptatretinae, differ in that the latter has muscle fibers embedded in the skin. The resting position of the Pacific hagfish also tends to be coiled, while that of the Atlantic hagfish is stretched.[8][9]
Slime

Hagfish are long and vermiform, and can exude copious quantities of a milky and fibrous slime or mucus from some 100 glands or invaginations running along their flanks.[10] The species Myxine glutinosa was named for this slime. When captured and held, e.g., by the tail, they secrete the microfibrous mucus, which expands into up to 20 litres (5 1⁄4 US gallons) of sticky, gelatinous material when combined with water;[11] one litre of slime has about 40 milligrams of mucus and proteins.[12] If they remain captured, they can tie themselves in an overhand knot, which works its way from the head to the tail of the animal, scraping off the slime as it goes and freeing them from their captor. This singular behavior may assist them in extricating themselves from the jaws of predatory fish or from the interior of their own prey, and the sliming might act as a distraction to predators.[12]
Recently, the slime was reported to entrain water in its keratin-like intermediate filaments, creating a slow-to-dissipate, viscoelastic substance, rather than a simple gel. It has been proven to impair the function of a predator fish's gills. In this case, the hagfish's mucus would clog the predator's gills, disabling their ability to respire. The predator would release the hagfish to avoid suffocation. Because of the mucus, few marine predators target the hagfish. Other predators of hagfish are varieties of birds or mammals.[13]
Free-swimming hagfish also slime when agitated, and later clear the mucus using the same travelling-knot behavior.[14][15] The reported gill-clogging effect suggests that the travelling-knot behavior is useful or even necessary to restore the hagfish's own gill function after sliming.
Hagfish thread keratin (EsTKα and EsTKγ; Q90501 and Q90502), the protein that make up its slime filaments, is under investigation as an alternative to spider silk for use in applications such as body armor.[16] These alpha-keratin proteins in hagfish slime transform from an α-helical structure to a stiffer β sheet structure when stretched.[17] With combined draw-processing (stretching) and chemical crosslinking, recombinant slime keratin turns into a very strong fiber with stiffness reaching 20 GPa.[18]
Respiration
A hagfish generally respires by taking in water through its pharynx, past the velar chamber, and bringing the water through the internal gill pouches, which can vary in number from five to 16 pairs, depending on species.[19] The gill pouches open individually, but in Myxine, the openings have coalesced, with canals running backwards from each opening under the skin, uniting to form a common aperture on the ventral side known as the branchial opening. The esophagus is also connected to the left branchial opening, which is therefore larger than the right one, through a pharyngocutaneous duct (esophageocutaneous duct), which has no respiratory tissue. This pharyngocutaneous duct is used to clear large particles from the pharynx, a function also partly taking place through the nasopharyngeal canal. In other species, the coalescence of the gill openings is less complete, and in Bdellostoma, each pouch opens separately to the outside like in lampreys.[20][21] The unidirectional water flow passing the gills is produced by rolling and unrolling velar folds located inside a chamber developed from the nasohypophyseal tract, and is operated by a complex set of muscles inserting into cartilages of the neurocranium, assisted by peristaltic contractions of the gill pouches and their ducts.[22] Hagfish also have a well-developed dermal capillary network that supplies the skin with oxygen when the animal is buried in anoxic mud, as well as a high tolerance for both hypoxia and anoxia, with a well developed anaerobic metabolism.[23] The skin has also been suggested to be capable of cutaneous respiration.[24]
Nervous system
The origins of the vertebrate nervous system are of considerable interest to evolutionary biologists, and cyclostomes (hagfish and lampreys) are an important group for answering this question. The complexity of the hagfish brain has been an issue of debate since the late 19th century, with some morphologists believing that they do not possess a cerebellum, while others believe that it is continuous with the midbrain.[25] It is now believed that the hagfish neuroanatomy is similar to that of lampreys.[26] A common feature of both cyclostomes is the absence of myelin in neurons.[27]
Eye
The hagfish's eye, which lacks a lens, extraocular muscles, and the three motor cranial nerves (III, IV, and VI), is significant to the evolution of more complex eyes. A parietal eye and the parapineal organ are also absent in extant hagfish.[28][29] Hagfish eyespots, when present, can detect light, but as far as it is known, none can resolve detailed images. In Myxine and Neomyxine, the eyes are partly covered by the trunk musculature.[6] Paleontological evidence suggests, however, that the hagfish eye is not pleisiomorphic but rather degenerative, as fossils from the Carboniferous have revealed hagfish-like vertebrates with complex eyes. This would suggest that ancestrally Myxini possessed complex eyes.[30][31]
Cardiac function, circulation, and fluid balance
Hagfish are known to have one of the lowest blood pressures among the vertebrates.[32] One of the most primitive types of fluid balance found is among these creatures, whenever a rise in extracellular fluid occurs, the blood pressure rises and this, in turn, is sensed by the kidney, which excretes excess fluid.[23] They also have the highest blood volume to body mass of any chordate, with 17 ml of blood per 100 g of mass.[33]
The hagfish circulatory system has been of considerable interest to evolutionary biologists, who first believed that the hagfish heart was not innervated like that in jawed vertebrates.[34] Further investigation revealed that the hagfish did have a true innervated heart. The hagfish circulatory system also consists of multiple accessory pumps throughout the body, which are considered auxiliary “hearts”.[32]
Hagfish are the only known vertebrates with osmoregulation isosmotic to their external environment. Hypothetically, they excrete ions in bile salts.[35]
Musculoskeletal system
Hagfish musculature differs from jawed vertebrates in that they do not have a horizontal septum nor vertical septum, junctions of connective tissue that separate the hypaxial musculature and epaxial musculature. They do, however, have true myomeres and myosepta like all vertebrates. The mechanics of their craniofacial muscles in feeding have been investigated, revealing advantages and disadvantages of the dental plate. In particular, hagfish muscles have increased force and gape size compared to similar-sized jawed vertebrates, but lack the speed amplification, suggesting that jaws are faster acting.[36]
The hagfish skeleton comprises the skull, the notochord, and the caudal fin rays. The first diagram of the hagfish endoskeleton was made by Frederick Cole in 1905.[37] In Cole's monograph, he described sections of the skeleton that he termed "pseudo-cartilage", referring to its distinct properties compared to jawed chordates. The lingual apparatus of hagfish is composed of a cartilage base bearing two teeth-covered plates (dental plate) articulated with a series of large cartilage shafts. The nasal capsule is considerably expanded in hagfish, comprising a fibrous sheath lined with cartilage rings. In contrast to lampreys, the braincase is noncartilaginous. The role of the branchial arches is highly speculative, as hagfish embryos undergo a caudal shift of the posterior pharyngeal pouches; thus, the branchial arches do not support gills.[38] While parts of the hagfish skull are thought to be homologous with lampreys, they are thought to have very few homologous elements with jawed vertebrates.[39]
Reproduction
Very little is known about hagfish reproduction. Embryos are difficult to obtain for study, although laboratory breeding of the Far Eastern inshore hagfish, Eptatretus burgeri, has succeeded.[40] In some species, sex ratio has been reported to be as high as 100:1 in favor of females. Some hagfish species are thought to be hermaphroditic, having both an ovary and a testicle (only one gamete production organ is in both females and males). In some cases, the ovary is thought to remain nonfunctional until the individual has reached a particular age or encounters a particular environmental stress. These two factors in combination suggest the survival rate of hagfish is quite high.
Depending on species, females lay from one to 30 tough, yolky eggs. These tend to aggregate due to having Velcro-like tufts at either end. Hagfish are sometimes seen curled around small clutches of eggs. If this constitutes actual breeding behavior is uncertain.
Hagfish do not have a larval stage, in contrast to lampreys, which have a long one.
Hagfish have a mesonephric kidney and are often neotenic of their pronephric kidney. The kidney(s) are drained via mesonephric/archinephric duct. Unlike many other vertebrates, this duct is separate from the reproductive tract. Unlike all other vertebrates, the proximal tubule of the nephron is also connected with the coelom, provided lubrication.
The single testicle or ovary has no transportation duct. Instead, the gametes are released into the coelom until they find their way to the posterior end of the caudal region, whereby they find an opening in the digestive system.
The hagfish embryo can develop for as long as 11 months before hatching. This is retarded in comparison to other jawless vertebrates.[41] Information on their embryology has been obscured until recently, when husbandry advances enabled considerable insight into the group's evolutionary development. New insights into the evolution of neural crest cells, support the consensus that all vertebrates share these cells, which might be regulated by a common subset of genes.[42]
The inshore hagfish is the only member of the hagfish family with a seasonal reproductive cycle.[43]
Feeding
While polychaete marine worms on or near the sea floor are a major food source, hagfish can feed upon and often even enter and eviscerate the bodies of dead and dying/injured sea creatures much larger than themselves. They are known to devour their prey from the inside.[44] Hagfish have the ability to absorb dissolved organic matter across the skin and gill, which may be an adaptation to a scavenging lifestyle, allowing them to maximize sporadic opportunities for feeding. From an evolutionary perspective, hagfish represent a transitory state between the generalized nutrient absorption pathways of aquatic invertebrates and the more specialized digestive systems of aquatic vertebrates.[45]
Like leeches, they have a sluggish metabolism and can survive months between feedings;[46][47] their feeding behavior, however, appears quite vigorous. Analysis of the stomach content of several species has revealed a large variety of prey, including polychaetes, shrimp, hermit crabs, cephalopods, brittlestars, bony fishes, sharks, birds, and whale flesh.[48]
In captivity, hagfish are observed to use the overhand-knot behavior in reverse (tail-to-head) to assist them in gaining mechanical advantage to pull out chunks of flesh from carrion fish or cetaceans, eventually making an opening to permit entry to the interior of the body cavity of larger carcasses. A healthy larger sea creature likely would be able to outfight or outswim this sort of assault.
This energetic opportunism on the part of the hagfish can be a great nuisance to fishermen, as they can devour or spoil entire deep drag-netted catches before they can be pulled to the surface. Since hagfish are typically found in large clusters on and near the bottom, a single trawler's catch could contain several dozen or even hundreds of hagfish as bycatch, and all the other struggling, captive sea life make easy prey for them.
The digestive tract of the hagfish is unique among the chordates because the food in the gut is enclosed in a permeable membrane, analogous to the peritrophic matrix of insects.[49]
Hagfish have also been observed actively hunting the red bandfish, Cepola haastii, in its burrow, possibly using their slime to suffocate the fish before grasping it with their dental plates and dragging it from the burrow.[50]
Classification
Originally, Myxine was included by Linnaeus (1758) in Vermes. The fossil hagfish Myxinikela siroka from the Late Carboniferous of the United States is in some respects more similar to lampreys, but shows key autapomorphies of hagfish.[51] In recent years, hagfish have become of special interest for genetic analysis investigating the relationships among chordates. Their classification as agnathans places hagfish as elementary vertebrates in between invertebrates and gnathostomes. However, discussion has long occurred in scientific literature about whether the hagfish were even invertebrate. Using fossil data, paleontologists posited that lampreys are more closely related to gnathostomes than hagfish. The term “Craniata” was used to refer to animals that had a developed skull, but were not considered true vertebrates.[52] Molecular evidence in the early 1990s first began suggesting that lampreys and hagfish were more closely related to each other than to gnathostomes.[53] The validity of the taxon "Craniata" was further examined by Delarbre et al. (2002) using mtDNA sequence data, concluding the Myxini are more closely related to the Hyperoartia than to the Gnathostomata – i.e., that modern jawless fishes form a clade called the Cyclostomata. The argument is that if the Cyclostomata are indeed monophyletic, Vertebrata would return to its old content (Gnathostomata + Cyclostomata) and the name Craniata, being superfluous, would become a junior synonym.[5] Nowadays, molecular data are almost unanimously in consensus of cyclostome monophyly, with more recent work being directed at shared microRNAs between cyclostomes and gnathostomes.[54] The current classification supported by molecular analyses (which show that lampreys and hagfishes are sister taxa), as well as the fact that hagfishes do, in fact, have rudimentary vertebrae, which places hagfishes in Cyclostomata.[3]
Phylogeny
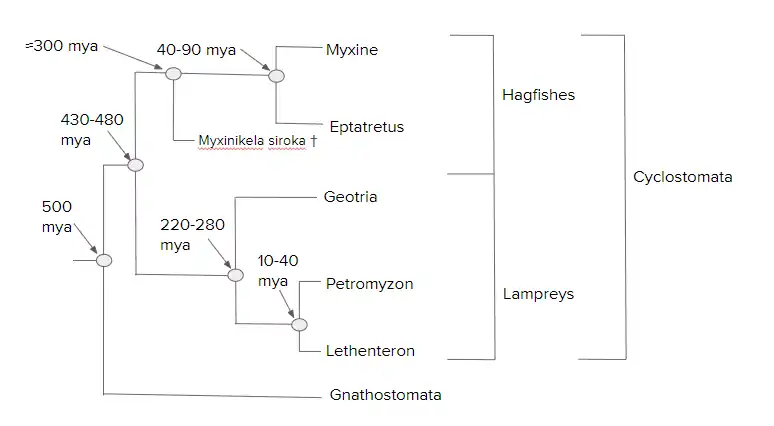
The following hagfish and lamprey phylogeny is an adaptation based on the 2006 work by Shigeru Kuratani and Shigehiro Kuraku:[55]
Commercial use

As food
In most of the world, hagfish are not often eaten. In Korea, the hagfish is a valued food, where it is generally skinned, coated in spicy sauce, and grilled over charcoal or stir-fried. It is especially popular in the southern port cities of the peninsula, such as Busan. The inshore hagfish, found in the Northwest Pacific, is eaten in Japan.[43] As hagfish slime binds vast amounts of liquid even at low temperatures, it was proposed as an energy-saving alternative for the production of tofu that does not require heating.[56]
In textiles
The hagfish slime threads can be used as ultra-strong fiber for clothing. Douglas Fudge, of Chapman University, has conducted research in this area.[57][58]
Skins
Hagfish skin, used in a variety of clothing accessories, is usually referred to as "eel skin". It produces a particularly durable leather, especially suitable for wallets and belts.[59]
References
- Nelson, Joseph S.; Grande, Terry C.; Wilson, Mark V. H. (2016). Fishes of the World (5th ed.). John Wiley & Sons. ISBN 9781118342336.
- van der Laan, Richard; Eschmeyer, William N.; Fricke, Ronald (2014). "Family-group names of Recent fishes". Zootaxa. 3882 (2): 001–230. doi:10.11646/zootaxa.3882.1.1. ISSN 1175-5326. PMID 25543675.
- Reece, Jane (2014). Campbell Biology. Boston: Pearson. p. 717. ISBN 978-0321775658.
- Myxini Archived 2017-12-15 at the Wayback Machine – University of California Museum of Paleontology
- Janvier, P. (2010). "MicroRNAs revive old views about jawless vertebrate divergence and evolution". Proceedings of the National Academy of Sciences. 107 (45): 19137–19138. Bibcode:2010PNAS..10719137J. doi:10.1073/pnas.1014583107. PMC 2984170. PMID 21041649.
Although I was among the early supporters of vertebrate paraphyly, I am impressed by the evidence provided by Heimberg et al. and prepared to admit that cyclostomes are, in fact, monophyletic. The consequence is that they may tell us little, if anything, about the dawn of vertebrate evolution, except that the intuitions of 19th century zoologists were correct in assuming that these odd vertebrates (notably, hagfishes) are strongly degenerate and have lost many characters over time
- Hyperotreti. Tree of Life
- The world's fastest shark is no match for a sack of flaccid hagfish skin
- How the slimy hagfish ties itself up in knots—and survives shark attacks
- Comparative Biomechanics of Hagfish Skins SICB - 2017 meeting - Abstract Details
- Rothschild, Anna (2013-04-01). "Hagfish slime: The clothing of the future?". BBC News. Retrieved 2013-04-02.
- "Snotties at Southern Encounter". Southern Encounter Aquarium and Kiwi House. 2007-10-30. Archived from the original on June 7, 2013. Retrieved 2008-10-30.
- Yong, Ed (2019-01-23). "No One Is Prepared for Hagfish Slime". The Atlantic. Retrieved 2019-01-26.
- Lim, J; Fudge, DS; Levy, N; Gosline, JM (January 31, 2006). "Hagfish slime ecomechanics: testing the gill-clogging hypothesis". Journal of Experimental Biology. 209 (Pt 4): 702–710. doi:10.1242/jeb.02067. PMID 16449564.
- Martini, F. H. (1998). "The ecology of hagfishes". In Jørgensen, J. M.; Lomholt, J. P.; Weber, R. E.; Malte, H. (eds.). The Biology of Hagfishes. London: Chapman and Hall. pp. 57–77. ISBN 978-0-412-78530-6.
- Strahan, R. (1963). "The behavior of myxinoids". Acta Zool. 44 (1–2): 73–102. doi:10.1111/j.1463-6395.1963.tb00402.x.
- "Slime from this 300 million-year-old creature could create bulletproof body armor". New York Post. 2017-10-25. Retrieved 2017-10-26.
- Fu, Jing; Guerette, Paul A.; Miserez, Ali (8 July 2015). "Self-Assembly of Recombinant Hagfish Thread Keratins Amenable to a Strain-Induced α-Helix to β-Sheet Transition". Biomacromolecules. 16 (8): 2327–2339. doi:10.1021/acs.biomac.5b00552. PMID 26102237.
- Fu, Jing; Guerette, Paul A.; Pavesi, Andrea; Horbelt, Nils; Lim, Chwee Teck; Harrington, Matthew J.; Miserez, Ali (2017). "Artificial hagfish protein fibers with ultra-high and tunable stiffness". Nanoscale. 9 (35): 12908–12915. doi:10.1039/c7nr02527k. PMID 28832693. Lay summary.
- Springer, Joseph; Holley, Dennis (2012). An Introduction to Zoology. Jones & Bartlett Publishers. pp. 376–. ISBN 978-1-4496-9544-6.
- Hughes, George Morgan (1963). Comparative Physiology of Vertebrate Respiration. Harvard University Press. pp. 9–. ISBN 978-0-674-15250-2.
- Wake, Marvalee H. (1992). Hyman's Comparative Vertebrate Anatomy. University of Chicago Press. pp. 81–. ISBN 978-0-226-87013-7.
- Bone, Quentin; Moore, Richard (2008). Biology of Fishes. Taylor & Francis. pp. 128–. ISBN 978-1-134-18631-0.
- Jørgensen, Jørgen Mørup (1998). The Biology of Hagfishes. Springer Science & Business Media. pp. 231–. ISBN 978-0-412-78530-6.
- Helfman, Gene; Collette, Bruce B.; Facey, Douglas E.; Bowen, Brian W. (2009). The Diversity of Fishes: Biology, Evolution, and Ecology. John Wiley & Sons. pp. 235–. ISBN 978-1-4443-1190-7.
- Larsell, O (1947), "The cerebellum of myxinoids and petromyzonts including developmental stages in the lampreys.", Journal of Experimental Biology, 210 (22): 3897–3909, doi:10.1002/cne.900860303, PMID 20239748
- Wicht, H (1996), "The brains of lampreys and hagfishes: Characteristics, characters, and comparisons.", Brain, Behavior and Evolution, 48 (5): 248–261, doi:10.1159/000113204, PMID 8932866
- Bullock, T.H.; Moore, J.K.; Fields, R.D. (1984). "Evolution of myelin sheaths: both lamprey and hagfish lack myelin". Neuroscience Letters. 48 (2): 145–148. doi:10.1016/0304-3940(84)90010-7. PMID 6483278.
- Ostrander, Gary Kent (2000). The Laboratory Fish. Elsevier. pp. 129–. ISBN 978-0-12-529650-2.
- "Keeping an eye on evolution". PhysOrg.com. 2007-12-03. Retrieved 2007-12-04.
- Gabbott, S.E; Donoghu, P.C; et al. (2016), "Pigmented anatomy in Carboniferous cyclostomes and the evolution of the vertebrate eye.", Proc. R. Soc. B, 283 (1836): 20161151, doi:10.1098/rspb.2016.1151, PMC 5013770, PMID 27488650
- Bardack, D (1991), "First fossil hagfish (Myxinoidea): a record from the Pennsylvanian of Illinois", Science, 254 (5032): 701–3, Bibcode:1991Sci...254..701B, doi:10.1126/science.254.5032.701, PMID 17774799
- Forster, Malcolm E.; Axelsson, Michael; Farrell, Anthony P.; Nilsson, Stefan (1991-07-01). "Cardiac function and circulation in hagfishes". Canadian Journal of Zoology. 69 (7): 1985–1992. doi:10.1139/z91-277. ISSN 0008-4301.
- Hagfish - Cronodon
- Jensen, D (1965), "The aneural heart of the hagfish.", Annals of the New York Academy of Sciences, 127 (1): 443–58, Bibcode:1965NYASA.127..443J, doi:10.1111/j.1749-6632.1965.tb49418.x, PMID 5217274
- Robertson, J.D (1976), "Chemical composition of the body fluids and muscle of the hagfish Myxine glutinosa and the rabbit‐fish Chimaera monstros.", Journal of Zoology, 178 (2), doi:10.1111/j.1469-7998.1976.tb06012.x
- Clark, A.J.; Summers, A.P. (2007). "Morphology and kinematics of feeding in hagfish: possible functional advantages of jaws". Journal of Experimental Biology. 210 (22): 3897–3909. doi:10.1242/jeb.006940. PMID 17981857.
- Cole, F.J. (1906), "A Monograph on the general Morphology of the Myxinoid Fishes, based on a study of Myxine. Part I. The Anatomy of the Skeleton.", Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 41 (3)
- Oisi, Y.; Fujimoto, S.; Ota, K.G.; Kuratani, S. (2015). "On the peculiar morphology and development of the hypoglossal, glossopharyngeal and vagus nerves and hypobranchial muscles in the hagfish". Zoological Letters. 1 (1): 6. doi:10.1186/s40851-014-0005-9. PMC 4604111. PMID 26605051.

- Oisi, Y.; Ota, K.G.; Fujimoto, S.; Kuratani, S. (2013). "Development of the chondrocranium in hagfishes, with special reference to the early evolution of vertebrates". Zoological Science. 30 (11): 944–961. doi:10.2108/zsj.30.944. PMID 24199860.
- Holland, ND (2007). "Hagfish embryos again: The end of a long drought". BioEssays. 29 (9): 833–6. doi:10.1002/bies.20620. PMID 17691082.
- Gorbman, A (1997). "Hagfish development". Zoological Science. 14 (3): 375–390. doi:10.2108/zsj.14.375.
- Ota, K.G; Kuraku, S.; Kuratani, S. (2007). "Hagfish embryology with reference to the evolution of the neural crest". Nature. 446 (7136): 672–5. Bibcode:2007Natur.446..672O. doi:10.1038/nature05633. PMID 17377535.
- Froese, Rainer. "Epatretus burgeri Inshore hagfish". Fishbase. Retrieved 18 April 2019.
- Wilson, Hugh (November 2009) Hagfish – World's weirdest animals. green.ca.msn.com
- Glover, CN; Bucking, C; Wood, CM (2011-03-02). "Adaptations to in situ feeding: novel nutrient acquisition pathways in an ancient vertebrate". Proceedings of the Royal Society B: Biological Sciences. 278 (1721): 3096–101. doi:10.1098/rspb.2010.2784. PMC 3158932. PMID 21367787.
- "Introduction to the Myxini". Berkeley.edu website. Archived from the original on 2017-12-15. Retrieved 2009-01-25.
- Lesser, M; Martini, Frederic H.; Heiser, John B. (3 January 1997). "Ecology of the hagfish, Myxine glutinosa L. in the Gulf of Maine I. Metabolic rates and energetics". Journal of Experimental Marine Biology and Ecology. 208 (1–2): 215–225. doi:10.1016/S0022-0981(96)02665-2.
- Zintzen, V.; Rogers, K. M.; Roberts, C. D.; Stewart, A. L.; Anderson, M. J. (2013). "Hagfish feeding habits along a depth gradient inferred from stable isotopes" (PDF). Marine Ecology Progress Series. 485: 223–234. Bibcode:2013MEPS..485..223Z. doi:10.3354/meps10341.
- Piper, Ross (2007), Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals, Greenwood Press.
- Zintzen, V.; Roberts, C. D.; Anderson, M. J.; Stewart, A. L.; Struthers, C. D.; Harvey, E. S. (2011). "Hagfish predatory behaviour and slime defence mechanism". Scientific Reports. 1: 131. Bibcode:2011NatSR...1E.131Z. doi:10.1038/srep00131. PMC 3216612. PMID 22355648.
- Miyashita, Tetsuto (23 November 2020). "A Paleozoic stem hagfish Myxinikela siroka — revised anatomy and implications for evolution of the living jawless vertebrate lineages". Canadian Journal of Zoology. 98 (12): 850–865. doi:10.1139/cjz-2020-0046. ISSN 0008-4301.
- Forey, P.; Janvier, P. (1993). "Agnathans and the origin of jawed vertebrates". Nature. 361 (6408): 129–134. Bibcode:1993Natur.361..129F. doi:10.1038/361129a0.
- Stock, D.W.; Whitt, G.S. (1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group". Science. 257 (5071): 787–9. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- Heimberg, A.M; et al. (2010). "microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate". Proceedings of the National Academy of Sciences. 107 (45): 19379–83. doi:10.1073/pnas.1010350107. PMC 2984222. PMID 20959416.
- Kuraku, S.; Kuratani, S. (2006). "Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences". Zoological Science. 23 (12): 1053–1064. doi:10.2108/zsj.23.1053. PMID 17261918.
- Böni, Lukas; Rühs, Patrick A.; Windhab, Erich J.; Fischer, Peter; Kuster, Simon (25 January 2016). "Gelation of Soy Milk with Hagfish Exudate Creates a Flocculated and Fibrous Emulsion- and Particle Gel". PLOS One. 11 (1): e0147022. Bibcode:2016PLoSO..1147022B. doi:10.1371/journal.pone.0147022. PMC 4726539. PMID 26808048.
- Say hello to fish slime bulletproof vests
- Guelph Research
- Dillman, Terry (1 February 2013). "Slimed: Ugly Hagfish Yields Somewhat Pretty Income". Fishermen's News.
Further reading
- Froese, Rainer, and Daniel Pauly, eds. (2011). "Myxinidae" in FishBase. February 2011 version.
- Bardack, D (1991). "First fossil hagfish (Myxinoidea): a record from the Pennsylvanian of Illinois". Science. 254 (5032): 701–703. Bibcode:1991Sci...254..701B. doi:10.1126/science.254.5032.701. PMID 17774799.
- Bardack, D.; Richardson, E. S. Jr (1977). "New agnathous fishes from the Pennsylvanian of Illinois". Fieldiana. Geology. 33: 489–510. doi:10.5962/bhl.title.5167.
- Brodal, A. and Fänge, R. (ed.) (1963). The Biology of Myxine, Universitetsforlaget, Oslo.
- Fernholm, B.; Holmberg, K. (1975). "The eyes in three genera of hagfish (Eptatretus, Paramyxine and Myxine) – A case of degenerative evolution". Vision Research. 15 (2): 253–259. doi:10.1016/0042-6989(75)90215-1. PMID 1129982.
- Hardisty, M. W. (1982). Lampreys and hagfishes: Analysis of cyclostome relationships. In The Biology of Lampreys, (ed. M. W. Hardisty and I. C. Potter), Vol.4B, pp. 165–259. Academic Press, London.
- Janvier, P. (1996). Early vertebrates. Oxford Monographs in Geology and Geophysics, 33, Oxford University Press, Oxford.
- Marinelli, Wilhelm (1956). Vergleichende Anatomie und Morphologie der Wirbeltiere: 2. Lieferung. Myxine glutinosa (L.). Franz Deuticke.
- Yalden, D.W. (1985). "Feeding mechanisms as evidence for cyclostome monophyly". Zoological Journal of the Linnean Society. 84 (3): 291–300. doi:10.1111/j.1096-3642.1985.tb01802.x.
- Stock, D. W.; Whitt, G. S. (1992). "Evidence from 18S ribosomal RNA that lampreys and hagfishes form a natural group". Science. 257 (5071): 787–789. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- Mincarone, Michael M.; Stewart, Andrew L. (2006). "A new species of giant seven-gilled hagfish (Myxinidae: Eptatretus) from New Zealand". Copeia. 2006 (2): 225–229. doi:10.1643/0045-8511(2006)6[225:ANSOGS]2.0.CO;2.
- J.M. Jørgensen; J.P. Lomholt; R.E. Weber; H. Malte, eds. (1997). The biology of hagfishes. London: Chapman & Hall.
- Delarbre, C; et al. (2002). "Complete Mitochondrial DNA of the Hagfish, Eptatretus burgeri: The Comparative Analysis of Mitochondrial DNA Sequences Strongly Supports the Cyclostome Monophyly". Molecular Phylogenetics and Evolution. 22 (2): 184–192. doi:10.1006/mpev.2001.1045. PMID 11820840.
- Bondareva & Schmidt, EE (November 2003). "Early Vertebrate Evolution of the TATA-Binding Protein, TBP". Molecular Biology and Evolution. 20 (11): 1932–1939. doi:10.1093/molbev/msg205. PMC 2577151. PMID 12885957.
- Ewoldt, R. H., Winegard, T. M. and Fudge D. S. (2010). Non-linear viscoelasticity of hagfish slime. Int. J. Lin. Mech. 46: 627–636.
- Fudge, D. (2001). Hagfishes: Champions of Slime Nature Australia, Spring 2001 ed., Australian Museum Trust, Sydney. pp. 61–69.
- Fudge, D. S.; Gardner, K. H.; Forsyth, V. T.; Riekel, C.; Gosline, J. M. (2003). "The mechanical properties of hydrated intermediate filaments: Insights from hagfish gland thread cells". Biophysical Journal. 85 (3): 2015–2027. Bibcode:2003BpJ....85.2015F. doi:10.1016/S0006-3495(03)74629-3. PMC 1303373. PMID 12944314.
- Fudge, D. S.; Hillis, S.; Levy, N.; Gosline, J. M. (2010). "Hagfish slime threads as a biomimetic model for high performance protein fibres" (PDF). Bioinspiration & Biomimetics. 5 (3): 1–8. Bibcode:2010BiBi....5c5002F. doi:10.1088/1748-3182/5/3/035002. PMID 20729569.
- Fudge, D. S.; Levy, N.; Chiu, S.; Gosline, J. M. (2005). "Composition, morphology and mechanics of hagfish slime". Journal of Experimental Biology. 208 (24): 4613–4625. doi:10.1242/jeb.01963. PMID 16326943.
- Winegard, T. M.; Fudge, D. S. (2010). "Deployment of hagfish slime thread skeins requires the transmission of mixing forces via mucin strands". Journal of Experimental Biology. 213 (8): 1235–1240. doi:10.1242/jeb.038075. PMID 20348334.
External links
| Wikimedia Commons has media related to Myxinidae. |
| Wikisource has the text of the 1905 New International Encyclopedia article "Hagfish". |
- FishBase entry for Myxinidae
- YouTube 5+ minute video of Scripps scientist/diver on hagfish
- Metacafe video of a University of Alberta grad student showing slime production of hagfish while in Bamfield, British Columbia
- Beware the hagfish – repeller of sharks 3 News, 28 Oct 2011. Video.
- Hagfish versus sharks : 1-0 Te Papa Blog, 28 October 2011.
- Teen Spots Hagfish-Slurping Elephant Seal – YouTube (2:11)
- What happens when a shark attacks a hagfish – BBC (0:39)
- Vancouver Aquarium Hagfish Slime