Hydroxychloroquine
Hydroxychloroquine is a medication used to prevent and treat malaria in areas where malaria remains sensitive to chloroquine. Other uses include treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda. It is taken by mouth, often in the form of hydroxychloroquine sulfate.[2]
 Skeletal formula of hydroxychloroquine | |
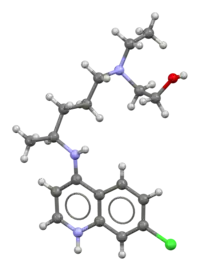 Ball-and-stick model of the hydroxychloroquine freebase molecule | |
| Clinical data | |
|---|---|
| Trade names | Plaquenil, others |
| Other names | HCQ |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601240 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Variable (74% on average); Tmax = 2–4.5 hours |
| Protein binding | 45% |
| Metabolism | Liver |
| Elimination half-life | 32–50 days |
| Excretion | Mostly kidney (23–25% as unchanged drug), also biliary (<10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.864 |
| Chemical and physical data | |
| Formula | C18H26ClN3O |
| Molar mass | 335.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects may include vomiting, headache, changes in vision, and muscle weakness.[2] Severe side effects may include allergic reactions, vision problems, and heart problems.[2][3] Although all risk cannot be excluded, it remains a treatment for rheumatic disease during pregnancy.[4] Hydroxychloroquine is in the antimalarial and 4-aminoquinoline families of medication.[2]
Hydroxychloroquine was approved for medical use in the United States in 1955.[2] It is on the World Health Organization's List of Essential Medicines.[5] In 2017, it was the 128th most commonly prescribed medication in the United States, with more than five million prescriptions.[6][7] Hydroxychloroquine is being studied to prevent and treat coronavirus disease 2019 (COVID‑19), but all clinical trials conducted during 2020 found it is ineffective and may cause dangerous side effects.[8][9][10][11] The speculative use of hydroxychloroquine for COVID‑19 threatens its availability for people with established indications.[9]
Medical uses
Hydroxychloroquine treats rheumatic disorders such as systemic lupus erythematosus, rheumatoid arthritis, and porphyria cutanea tarda, and certain infections such as Q fever and certain types of malaria.[2] It is considered the first-line treatment for systemic lupus erythematosus.[12] Certain types of malaria, resistant strains, and complicated cases require different or additional medication.[2]
It is widely used to treat primary Sjögren syndrome but does not appear to be effective.[13] Hydroxychloroquine is widely used in the treatment of post-Lyme arthritis. It may have both an anti-spirochete activity and an anti-inflammatory activity, similar to the treatment of rheumatoid arthritis.[14]
Contraindications
The drug label advises that hydroxychloroquine should not be prescribed to individuals with known hypersensitivity to 4-aminoquinoline compounds.[15] There are several other contraindications,[16][17] and caution is required if the person considered for treatment has certain heart conditions, diabetes, or psoriasis.
Adverse effects
The most common adverse effects are nausea, stomach cramps, and diarrhea. Other common adverse effects include itching and headache.[9] The most serious adverse effects affect the eye, with dose-related retinopathy as a concern even after hydroxychloroquine use is discontinued.[2] Serious reported neuropsychiatric adverse effects of hydroxychloroquine use include agitation, mania, difficulty sleeping, hallucinations, psychosis, catatonia, paranoia, depression, and suicidal thoughts.[9] In rare situations, hydroxychloroquine has been implicated in cases of serious skin reactions such as Stevens–Johnson syndrome, toxic epidermal necrolysis, and Drug reaction with eosinophilia and systemic symptoms.[9] Reported blood abnormalities with its use include lymphopenia, eosinophilia, and atypical lymphocytosis.[9]
For short-term treatment of acute malaria, adverse effects can include abdominal cramps, diarrhea, heart problems, reduced appetite, headache, nausea and vomiting.[2] Other adverse effects noted with short-term use of Hydroxychloroquine include low blood sugar and QT interval prolongation.[8] Idiosyncratic hypersensitivity reactions have occurred.[9]
For prolonged treatment of lupus or rheumatoid arthritis, adverse effects include the acute symptoms, plus altered eye pigmentation, acne, anemia, bleaching of hair, blisters in mouth and eyes, blood disorders, cardiomyopathy,[8] convulsions, vision difficulties, diminished reflexes, emotional changes, excessive coloring of the skin, hearing loss, hives, itching, liver problems or liver failure, loss of hair, muscle paralysis, weakness or atrophy, nightmares, psoriasis, reading difficulties, tinnitus, skin inflammation and scaling, skin rash, vertigo, weight loss, and occasionally urinary incontinence.[2] Hydroxychloroquine can worsen existing cases of both psoriasis and porphyria.[2]
Children may be especially vulnerable to developing adverse effects from hydroxychloroquine overdoses.[2]
Eyes
One of the most serious side effects is retinopathy (generally with chronic use).[2][18] People taking 400 mg of hydroxychloroquine or less per day generally have a negligible risk of macular toxicity, whereas the risk begins to increase when a person takes the medication over five years or has a cumulative dose of more than 1000 grams. The daily safe maximum dose for eye toxicity can be estimated from a person's height and weight.[19] Macular toxicity is related to the total cumulative dose rather than the daily dose. Regular eye screening, even in the absence of visual symptoms, is recommended to begin when either of these risk factors occurs.[20]
Toxicity from hydroxychloroquine may be seen in two distinct areas of the eye: the cornea and the macula. The cornea may become affected (relatively commonly) by an innocuous cornea verticillata or vortex keratopathy and is characterized by whorl-like corneal epithelial deposits. These changes bear no relationship to dosage and are usually reversible on cessation of hydroxychloroquine.
The macular changes are potentially serious. Advanced retinopathy is characterized by reduction of visual acuity and a "bull's eye" macular lesion which is absent in early involvement.
Overdose
Overdoses of hydroxychloroquine are extremely rare, but extremely toxic.[9] Eight people are known to have overdosed since the drug's introduction in the mid-1950s, of which three have died.[21][22] Chloroquine has a risk of death in overdose in adults of about 20%, while hydroxychloroquine is estimated to be two or threefold less toxic.[23]
Serious signs and symptoms of overdose generally occur within an hour of ingestion.[23] These may include sleepiness, vision changes, seizures, coma, stopping of breathing, and heart problems such as ventricular fibrillation and low blood pressure.[9][23][24] Loss of vision may be permanent.[25] Low blood potassium, to levels of 1 to 2 mmol/L, may also occur.[23][26] Cardiovascular abnormalities such as QRS complex widening and QT interval prolongation may also occur.[9]
Treatment recommendations include early mechanical ventilation, heart monitoring, and activated charcoal.[23] Supportive treatment with intravenous fluids and vasopressors may be required with epinephrine being the vasopressor of choice.[23] Stomach pumping may also be used.[21] Sodium bicarbonate and hypertonic saline may be used in cases of severe QRS complex widening.[9] Seizures may be treated with benzodiazepines.[23] Intravenous potassium chloride may be required, however this may result in high blood potassium later in the course of the disease.[23] Dialysis does not appear to be useful.[23]
Detection
Hydroxychloroquine may be quantified in plasma or serum to confirm a diagnosis of poisoning in hospitalized victims or in whole blood to assist in a forensic investigation of a case of sudden or unexpected death. Plasma or serum concentrations are usually in a range of 0.1-1.6 mg/L during therapy and 6–20 mg/L in cases of clinical intoxication, while blood levels of 20–100 mg/L have been observed in deaths due to acute overdosage.[27]
Interactions
The drug transfers into breast milk.[1] There is no evidence that its use during pregnancy is harmful to the developing fetus and its use is not contraindicated in pregnancy.[9]
The concurrent use of hydroxychloroquine and the antibiotic azithromycin appears to increase the risk for certain serious side effects with short-term use, such as an increased risk of chest pain, congestive heart failure, and mortality from cardiovascular causes.[8] Care should be taken if combined with medication altering liver function as well as aurothioglucose (Solganal), cimetidine (Tagamet) or digoxin (Lanoxin). Hydroxychloroquinecan increase plasma concentrations of penicillamine which may contribute to the development of severe side effects. It enhances hypoglycemic effects of insulin and oral hypoglycemic agents. Dose altering is recommended to prevent profound hypoglycemia. Antacids may decrease the absorption of hydroxychloroquine. Both neostigmine and pyridostigmine antagonize the action of hydroxychloroquine.[28]
While there may be a link between hydroxychloroquine and hemolytic anemia in those with glucose-6-phosphate dehydrogenase deficiency, this risk may be low in those of African descent.[29]
Specifically, the US Food and Drug Administration's (FDA) drug label for hydroxychloroquine lists the following drug interactions:[15]
- Digoxin (wherein it may result in increased serum digoxin levels)
- Insulin or anti-diabetic medication (wherein it may enhance the effects of a hypoglycemic treatment)
- Drugs that prolong QT interval and other arrhythmogenic drugs (as Hydroxychloroquine prolongs the QT interval and may increase the risk of inducing serious abnormal heart rhythms (ventricular arrhythmias) if used concurrently)[3]
- Mefloquine and other drugs known to lower the seizure threshold (co-administration with other antimalarials known to lower the convulsion threshold may increase risk of convulsions)
- Antiepileptics (concurrent use may impair the antiepileptic activity)
- Methotrexate (combined use is unstudied and may increase the frequency of side effects)
- Cyclosporin (wherein an increased plasma cyclosporin level was reported when used together).
Pharmacology
Pharmacokinetics
Hydroxychloroquine has similar pharmacokinetics to chloroquine, with rapid gastrointestinal absorption, large distribution volume,[30] and elimination by the kidneys. Cytochrome P450 enzymes (CYP2D6, 2C8, 3A4 and 3A5) metabolize hydroxychloroquine to N-desethylhydroxychloroquine.[31] Both agents also inhibit CYP2D6 activity and may interact with other medications that depend on this enzyme.[9]
Pharmacodynamics
Antimalarials are lipophilic weak bases and easily pass plasma membranes. The free base form accumulates in lysosomes (acidic cytoplasmic vesicles) and is then protonated,[32] resulting in concentrations within lysosomes up to 1000 times higher than in culture media. This increases the pH of the lysosome from four to six.[33] Alteration in pH causes inhibition of lysosomal acidic proteases causing a diminished proteolysis effect.[34] Higher pH within lysosomes causes decreased intracellular processing, glycosylation and secretion of proteins with many immunologic and nonimmunologic consequences.[35] These effects are believed to be the cause of a decreased immune cell functioning such as chemotaxis, phagocytosis and superoxide production by neutrophils.[36] Hydroxychloroquine is a weak diprotic base that can pass through the lipid cell membrane and preferentially concentrate in acidic cytoplasmic vesicles. The higher pH of these vesicles in macrophages or other antigen-presenting cells limits the association of autoantigenic (any) peptides with class II MHC molecules in the compartment for peptide loading and/or the subsequent processing and transport of the peptide-MHC complex to the cell membrane.[37]
Mechanism of action
Hydroxychloroquine increases[38] lysosomal pH in antigen-presenting cells[8] by two mechanisms: As a weak base, it is a proton acceptor and via this chemical interaction, its accumulation in lysozymes raises the intralysosomal pH, but this mechanism does not fully account for the effect of hydroxychloroquine on pH. Additionally, in parasites that are susceptible to hydroxychloroquine, it interferes with the endocytosis and proteolysis of hemoglobin and inhibits the activity of lysosomal enzymes, thereby raising the lysosomal pH by more than 2 orders of magnitude over the weak base effect alone.[39][40] In 2003, a novel mechanism was described wherein hydroxychloroquine inhibits stimulation of the toll-like receptor (TLR) 9 family receptors. TLRs are cellular receptors for microbial products that induce inflammatory responses through activation of the innate immune system.[41]
As with other quinoline antimalarial drugs, the antimalarial mechanism of action of quinine has not been fully resolved. The most accepted model is based on hydrochloroquinine and involves the inhibition of hemozoin biocrystallization, which facilitates the aggregation of cytotoxic heme. Free cytotoxic heme accumulates in the parasites, causing death.[42]
Hydroxychloroquine increases the risk of low blood sugar through several mechanisms. These include decreased clearance of the hormone insulin from the blood, increased insulin sensitivity, and increased release of insulin from the pancreas.[9]
Hydroxychloroquine has been suggested to be a zinc ionophore, and may derive an anti-cancer action from increasing intracellular zinc uptake.[43]
Hydroxychloroquine can decrease tumor growth by cutting off the fuels that promote mitochondrial function. There are multiple phase I and II trials testing the efficacy of hydroxychloroquine.[44]
Society and culture
Brand names
It is frequently sold as a sulfate salt known as hydroxychloroquine sulfate.[2] 200 mg of the sulfate salt is equal to 155 mg of the base.[2]
Brand names of hydroxychloroquine include Plaquenil, Hydroquin, Axemal (in India), Dolquine, Quensyl, Quinoric.[45]
Misinformation versus science
Beginning in March 2020, US President Donald Trump began promoting hydroxychloroquine to prevent or treat COVID-19, citing small numbers of anecdotal reports.[46] Trump stated in June that he was taking the drug as a preventive measure,[47] stimulating unprecedented worldwide demand and causing shortages of hydroxychloroquine for its prescribed purpose of preventing malaria.[46]
After issuing an emergency use authorization for physicians to use the drug to treat hospitalized people with severe COVID-19 infection, the US Food and Drug Administration withdrew the authorization in June after finding hydroxychloroquine was unlikely to be effective and had serious side effects.[48] During ensuing months, additional studies found the drug was not effective,[49] and in late July, Anthony Fauci stated, "We know that every single good study — and by good study I mean randomized controlled study in which the data are firm and believable — has shown that hydroxychloroquine is not effective in the treatment of COVID-19."[11]
Research
COVID-19
Hydroxychloroquine is not effective for preventing coronavirus disease 2019 (COVID‑19) infection.[50] There is no strong scientific evidence to support the use of hydroxychloroquine as a COVID-19 treatment.[8][9][51][52] While its use is not approved by the FDA for COVID‑19 treatment, from April to June 2020, there was an emergency use authorization for its use in the United States,[53] and it has been used off label for potential treatment of the disease.[54] On 24 April 2020, citing the risk of "serious heart rhythm problems", the FDA posted a caution against using the drug for COVID‑19 "outside of the hospital setting or a clinical trial".[55] On 15 June, the FDA revoked its emergency use authorization, stating that it was "no longer reasonable to believe" that the drug was effective against COVID-19 or that its benefits outweighed "known and potential risks".[48][56][57][58]
Timeline
On 17 March 2020, Didier Raoult announced in an online video that a trial involving 24 patients from southeast France supported the claim that hydroxychloroquine and azithromycin were effective in treating for COVID-19.[59] On 20 March, he published a preliminary report of his study online in the International Journal of Antimicrobial Agents.[60] A later peer review found the study was “irresponsible.”[61]
On 17 March 2020, the AIFA Scientific Technical Commission of the Italian Medicines Agency expressed a favorable opinion on including the off-label use of chloroquine and hydroxychloroquine for the treatment of COVID‑19.[62]
During a press briefing on 19 March 2020, Donald Trump, the President of the United States, promoted the drugs chloroquine and hydroxychloroquine as a potential treatment for COVID‑19.[63][64] Trump claimed that chloroquine had been "approved very, very quickly" by the US Food and Drug Administration (FDA) while discussing treatments for COVID‑19. The FDA later said it had not given approval for the drug to be used in treatment of COVID‑19,[65] but was now allowing chloroquine under compassionate use guidelines.[66][67] Trump's remarks led to a shortage of chloroquine and hydroxychloroquine in the United States and panic-buying in Africa and South Asia.[68][69]
In the United States in March 2020, several state pharmacy boards reported that some doctors and dentists were writing prescriptions for hydroxychloroquine and a related drug, chloroquine, to themselves, family members, and staff.[68][70] Sudden demand spikes caused by hospital use for severely ill COVID‑19 patients and prescriptions for prophylaxis have resulted in shortages; doctors have expressed concern that patients who have long taken hydroxychloroquine for other approved indications, like lupus and rheumatoid arthritis, will be unable to procure needed medicine.[68][71]
News outlets reported on 24 March 2020 that, in the state of Arizona, a man had died, leaving his wife in critical condition, as a result of ingesting a product containing chloroquine phosphate designed for treating sick fish. The couple believed the fish treatment could prevent them from contracting COVID‑19.[72] Although chloroquine phosphate is a related compound to hydroxychloroquine, unlike hydroxychloroquine it is used for the treatment of fish parasites and infections. It has a different chemical structure from chloroquine and hydroxychloroquine which, when used in humans and other animals, could result in death.[72][73]
In early April 2020, a Texas nursing home administered hydroxychloroquine to dozens of residents who had COVID-19. The facility's medical director, Dr. Robin Armstrong, acquired the drug through his connections to the Republican Party; he made the request through Texas Lt. Gov. Dan Patrick.[74]
On 28 March 2020, the FDA issued an emergency use authorization (EUA) to allow hydroxychloroquine sulfate and chloroquine phosphate products donated to the Strategic National Stockpile (SNS) to be distributed and used for certain people who are hospitalized with COVID‑19.[75][76] In anticipation of product shortages, the FDA issued product-specific guidance for chloroquine phosphate and for hydroxychloroquine sulfate for generic drug manufacturers.[77] On 24 April 2020, "due to risk of heart rhythm problems", the FDA cautioned the use of hydroxychloroquine or chloroquine for COVID‑19 outside of the hospital setting or a clinical trial.[55]
On 29 April 2020, based on a preprint retrospective cohort study posted 10 April 2020, on medRxiv of more than 320,000 individuals that were not infected with COVID‑19, but had been treated with a combination of both hydroxychloroquine and azithromycin, an article published in The FASEB Journal cautioned against this combination to treat patients with COVID‑19 outside the context of a clinical trial. This was due to safety concerns with this combination and lack of evidence showing benefit to combining these medications. However the article suggested the combination could be used to treat COVID‑19 patients if new strong scientific evidence showed that combination as beneficial, there is no alternative treatment available, and the patients are counselled and closely monitored.[8]
On 18 May 2020, Trump publicly stated that he was taking hydroxychloroquine combined with zinc and an initial dose of azithromycin during the COVID‑19 pandemic.[78]
Based on the results of a 22 May 2020 study published in The Lancet (which was retracted on 4 June 2020) [79][80][81] the World Health Organization suspended hydroxychloroquine from its global drug trials for COVID‑19 treatments on 26 May 2020, due to safety concerns in that study. It had previously enrolled 3,500 patients from 17 countries in the Solidarity Trial, which was the official name of its study of hydroxychloroquine against COVID‑19.[82] Due to the increased risks described in a subsequently retracted study, France, Italy and Belgium banned the use of hydroxychloroquine as a COVID‑19 treatment.[83][84][85] None of the results used as the basis of these bans involved outpatient treatment. The first results for outpatient clinical trials of hydroxychloroquine with azithromycin are expected in September 2020.[86]
On 29 May 2020, the EMA issued a public health statement reminding healthcare professionals to closely monitor people with COVID‑19 who are receiving hydroxychloroquine.[87]
On 3 June 2020, the WHO announced it would resume its global trial of hydroxychloroquine, after its data safety monitoring committee found there was no increased risk of death for COVID‑19 patients taking it.[88]
On 4 June 2020, an article about the treatment of COVID‑19 patients using hydroxychloroquine with or without macrolides that had been published in The Lancet on 22 May 2020, was retracted by its authors. Before its retraction it had claimed that hospitalized COVID‑19 patients treated with hydroxychloroquine or chloroquine (with or without a macrolide) did not benefit from the treatment, but instead were at greater risk of death. However, since its publication questions were raised about the accuracy of the data used in the article, prompting a third party review which resulted in the retraction of the article from the journal.[79][80][81]
On 5 June 2020, Peter Horby, Professor of Emerging Infectious Diseases and Global Health in the Nuffield Department of Medicine, University of Oxford, and chief investigator for a randomised trial on hydroxychloroquine, said: "the RECOVERY Trial has shown that hydroxychloroquine is not an effective treatment in patients hospitalised with COVID-19".[89]
On 15 June 2020, the FDA revoked the emergency use authorization for hydroxychloroquine and chloroquine.[48][90][57][58] The FDA also updated the fact sheets for the emergency use authorization of remdesivir, to warn that using chloroquine or hydroxychloroquine with remdesivir may reduce the antiviral activity of remdesivir.[91]
In January 2021, Oklahoma was still dealing with having bought large quantities of the drug which it no longer needed.[92] The episode has inspired at least one medical user of hydrocychloroquine to take the opportunity to share accurate medical information about it in general and in the context of disability justice.[93]
References
- "Hydroxychloroquine Use During Pregnancy". Drugs.com. 28 February 2020. Retrieved 21 March 2020.
- "Hydroxychloroquine Sulfate Monograph for Professionals". The American Society of Health-System Pharmacists. 20 March 2020. Archived from the original on 20 March 2020. Retrieved 20 March 2020.
- "Guidance on patients at risk of drug-induced sudden cardiac death from off-label COVID-19 treatments". newsnetwork.mayoclinic.org. 25 March 2020.
- Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. (September 2016). "BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids". Rheumatology. 55 (9): 1693–7. doi:10.1093/rheumatology/kev404. PMID 26750124.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "The Top 300 of 2020". ClinCalc. Retrieved 18 March 2020.
- "Hydroxychloroquine Sulfate – Drug Usage Statistics". ClinCalc. Retrieved 7 April 2020.
- Meyerowitz EA, Vannier AG, Friesen MG, Schoenfeld S, Gelfand JA, Callahan MV, et al. (May 2020). "Rethinking the role of hydroxychloroquine in the treatment of COVID-19". FASEB Journal. 34 (5): 6027–6037. doi:10.1096/fj.202000919. PMC 7267640. PMID 32350928.
- Juurlink DN (April 2020). "Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection". CMAJ. 192 (17): E450–E453. doi:10.1503/cmaj.200528. PMC 7207200. PMID 32269021.
- Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S (June 2020). "A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19". Journal of Critical Care. 57: 279–283. doi:10.1016/j.jcrc.2020.03.005. PMC 7270792. PMID 32173110.
- "Coronavirus: Hydroxychloroquine ineffective says Fauci". BBC News Online. 29 July 2020.
- Chew CY, Mar A, Nikpour M, Saracino AM (May 2020). "Hydroxychloroquine in dermatology: New perspectives on an old drug". The Australasian Journal of Dermatology. 61 (2): e150–e157. doi:10.1111/ajd.13168. PMID 31612996. S2CID 204703558.
- Wang SQ, Zhang LW, Wei P, Hua H (May 2017). "Is hydroxychloroquine effective in treating primary Sjogren's syndrome: a systematic review and meta-analysis". BMC Musculoskeletal Disorders. 18 (1): 186. doi:10.1186/s12891-017-1543-z. PMC 5427554. PMID 28499370.
- Steere AC, Angelis SM (October 2006). "Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis". Arthritis and Rheumatism. 54 (10): 3079–86. doi:10.1002/art.22131. PMID 17009226.
- "Plaquenil- hydroxychloroquine sulfate tablet". DailyMed. 3 January 2020. Retrieved 20 March 2020.
- "Plaquenil (hydroxychloroquine sulfate) dose, indications, adverse effects, interactions". pdr.net. Retrieved 19 March 2020.
- "Drugs & Medications". webmd.com. Retrieved 19 March 2020.
- Flach AJ (2007). "Improving the risk-benefit relationship and informed consent for patients treated with hydroxychloroquine". Transactions of the American Ophthalmological Society. 105: 191–4, discussion 195–7. PMC 2258132. PMID 18427609.
- "Plaquenil Risk Calculators". EyeDock. Retrieved 7 April 2020.
- Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF (February 2011). "Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy" (PDF). Ophthalmology. 118 (2): 415–22. doi:10.1016/j.ophtha.2010.11.017. PMID 21292109.
- Aronson JK (2015). Meyler's Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions. Elsevier. p. 261. ISBN 978-0-444-53716-4.
- K Marquardt, K, Albertson, T, Treatment of hydroxychloroquine overdose, Am J Emerg Med, Sep 2001; 19(5):420-4, doi: 10.1053/ajem.2001.25774, pubmed.ncbi.nlm.nih.gov/11555803/
- Ling Ngan Wong A, Tsz Fung Cheung I, Graham CA (February 2008). "Hydroxychloroquine overdose: case report and recommendations for management". European Journal of Emergency Medicine. 15 (1): 16–8. doi:10.1097/MEJ.0b013e3280adcb56. PMID 18180661. S2CID 41205035.
- Smith ER, Klein-Schwartz W (May 2005). "Are 1-2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers" (PDF). The Journal of Emergency Medicine. 28 (4): 437–43. doi:10.1016/j.jemermed.2004.12.011. PMID 15837026. Archived from the original (PDF) on 15 April 2020. Retrieved 15 April 2020.
- Roque MR, Foster CS (23 March 2020). "Chloroquine and Hydroxychloroquine Toxicity: Practice Essentials, Background, Pathophysiology". Medscape. Archived from the original on 8 April 2020. Retrieved 7 April 2020.
- Pillay VV (2012). Modern Medical Toxicology (PDF). Jaypee Brothers Publishers. p. 458. ISBN 978-93-5025-965-8.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 12th edition, Biomedical Publications, Foster City, CA, 2020, pp. 1024-1026.
- "Russian Register of Medicines: Plaquenil (hydroxychloroquine) Film-coated Tablets for Oral Use. Prescribing Information". rlsnet.ru (in Russian). Sanofi-Synthelabo. Archived from the original on 16 August 2016. Retrieved 14 July 2016.
- Mohammad S, Clowse ME, Eudy AM, Criscione-Schreiber LG (March 2018). "Examination of Hydroxychloroquine Use and Hemolytic Anemia in G6PDH-Deficient Patients". Arthritis Care & Research. 70 (3): 481–485. doi:10.1002/acr.23296. PMID 28556555.
- Schrezenmeier E, Dörner T (March 2020). "Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology". Nature Reviews. Rheumatology. 16 (3): 155–166. doi:10.1038/s41584-020-0372-x. PMID 32034323. S2CID 211053837.
- Kalia S, Dutz JP (2007). "New concepts in antimalarial use and mode of action in dermatology". Dermatologic Therapy. 20 (4): 160–74. doi:10.1111/j.1529-8019.2007.00131.x. PMC 7163426. PMID 17970883.
- Kaufmann AM, Krise JP (April 2007). "Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications". Journal of Pharmaceutical Sciences. 96 (4): 729–46. doi:10.1002/jps.20792. PMID 17117426.
- Ohkuma S, Poole B (July 1978). "Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents". Proceedings of the National Academy of Sciences of the United States of America. 75 (7): 3327–31. Bibcode:1978PNAS...75.3327O. doi:10.1073/pnas.75.7.3327. PMC 392768. PMID 28524.
- Ohkuma S, Chudzik J, Poole B (March 1986). "The effects of basic substances and acidic ionophores on the digestion of exogenous and endogenous proteins in mouse peritoneal macrophages". The Journal of Cell Biology. 102 (3): 959–66. doi:10.1083/jcb.102.3.959. PMC 2114118. PMID 3949884.
- Oda K, Koriyama Y, Yamada E, Ikehara Y (December 1986). "Effects of weakly basic amines on proteolytic processing and terminal glycosylation of secretory proteins in cultured rat hepatocytes". The Biochemical Journal. 240 (3): 739–45. doi:10.1042/bj2400739. PMC 1147481. PMID 3493770.
- Hurst NP, French JK, Gorjatschko L, Betts WH (January 1988). "Chloroquine and hydroxychloroquine inhibit multiple sites in metabolic pathways leading to neutrophil superoxide release". The Journal of Rheumatology. 15 (1): 23–7. PMID 2832600.
- Fox R (June 1996). "Anti-malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development". Lupus. 5 Suppl 1: S4–10. doi:10.1177/0961203396005001031. PMID 8803903. S2CID 208217074.
- Waller D, Sampson T. Medical Pharmacology and Therapeutics (2nd ed.). p. 370.
- Krogstad DJ, Schlesinger PH (March 1987). "The basis of antimalarial action: non-weak base effects of chloroquine on acid vesicle pH". Am J Trop Med Hyg. 36 (2): 213–20. doi:10.4269/ajtmh.1987.36.213. PMID 2435182.
- Al-Bari MA (February 2017). "Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases". Pharmacol Res Perspect. 5 (1): e00293. doi:10.1002/prp2.293. PMC 5461643. PMID 28596841.
- Takeda K, Kaisho T, Akira S (2003). "Toll-like receptors". Annual Review of Immunology. 21: 335–76. doi:10.1146/annurev.immunol.21.120601.141126. PMID 12524386.
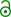
- Sullivan DJ (December 2002). "Theories on malarial pigment formation and quinoline action". International Journal for Parasitology. 32 (13): 1645–53. doi:10.1016/S0020-7519(02)00193-5. PMID 12435449.
- Xue, Jing; Moyer, Amanda; Peng, Bing; Wu, Jinchang; Hannafon, Bethany N.; Ding, Wei-Qun (1 October 2014). "Chloroquine Is a Zinc Ionophore". PLOS ONE. 9 (10): e109180. Bibcode:2014PLoSO...9j9180X. doi:10.1371/journal.pone.0109180. ISSN 1932-6203. PMC 4182877. PMID 25271834.
- Chandel, Navdeep S. (29 March 2019). "A New Way to Fight Cancer". Scientific American. Retrieved 21 August 2020.
- "Hydroxychloroquine trade names". Drugs-About.com. Retrieved 18 June 2019.
- Piller, Charles (26 March 2020). "'This is insane!' Many scientists lament Trump's embrace of risky malaria drugs for coronavirus". Science. doi:10.1126/science.abb9021. Retrieved 4 August 2020.
- Lovelace, Jr, Berkeley (15 June 2020). "FDA revokes emergency use of hydroxychloroquine". CNBC.
- "Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Retrieved 15 June 2020.
- "No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19". Recovery Trial, Nuffield Department of Population Health, University of Oxford, UK. 5 June 2020. Retrieved 7 June 2020.
- Smit M, Marinosci A, Agoritsas T, Ford N, Calmy A (January 2021). "Prophylaxis for COVID-19: a systematic review". Clin Microbiol Infect (Systematic review). doi:10.1016/j.cmi.2021.01.013. PMID 33476807.
- "Assessment of Evidence for COVID-19-Related Treatments: Updated 4/3/2020". ASHP. Retrieved 7 April 2020.
- Yazdany, J; Kim, AHJ (March 2020). "Use of Hydroxychloroquine and Chloroquine During the COVID-19 Pandemic: What Every Clinician Should Know". Annals of Internal Medicine. 172 (11): M20-1334. doi:10.7326/M20-1334. PMC 7138336. PMID 32232419.
- "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 11 February 2020. Retrieved 9 April 2020.
- Kalil AC (March 2020). "Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics". JAMA. 323 (19): 1897–1898. doi:10.1001/jama.2020.4742. PMID 32208486.
- "FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems". U.S. Food and Drug Administration (FDA). 24 April 2020.
- Berkeley Lovelace Jr (15 June 2020). "FDA revokes emergency use of hydroxychloroquine". CNBC.
- "HCQ and CQ revocation letter" (PDF). U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
- "Frequently Asked Questions on the Revocation of the Emergency Use Authorization for Hydroxychloroquine Sulfate and Chloroquine Phosphate" (PDF). U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
- France, Connexion. "French researcher posts successful Covid-19 drug trial". connexionfrance.com. Retrieved 18 March 2020.
- Gautret, Philippe; et al. (20 March 2020) [Online ahead of print]. "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial". International Journal of Antimicrobial Agents. 56 (1): 105949. doi:10.1016/j.ijantimicag.2020.105949. PMC 7102549. PMID 32205204.
- "Hydroxychloroquine: Europe Turns Away From Doctor Who Championed Drug With 'Irresponsible' Study". 21 July 2020.
- "Azioni intraprese per favorire la ricerca e l'accesso ai nuovi farmaci per il trattamento del COVID-19". Italian Medicines Agency (AIFA) (in Italian). 17 March 2020. Retrieved 18 March 2020.
- Nisen M (19 March 2020). "Trump Is Overhyping Unproven Coronavirus Drugs". The Washington Post. Bloomberg. Retrieved 24 March 2020.
- "Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing". White House. Retrieved 24 March 2020.
- Dale D (20 March 2020). "Fact check: Trump wrongly claims FDA 'approved' drug chloroquine to treat the coronavirus". CNN. Retrieved 29 March 2020.
- Naftulin J (20 March 2020). "The FDA is allowing two drugs to be used for 'compassionate use' to treat the coronavirus. Here's what that means". Business Insider. Retrieved 1 April 2020.
- Coppock K (19 March 2020). "FDA Announces Two Drugs Given 'Compassionate Use' Status in Treating COVID-19". Pharmacy Times. Retrieved 1 April 2020.
- Rowland C (23 March 2020). "As Trump touts an unproven coronavirus treatment, supplies evaporate for patients who need those drugs". The Washington Post. Retrieved 24 March 2020.
- Parkinson J, Gauthier-Villars D (23 March 2020). "Trump Claim That Malaria Drugs Treat Coronavirus Sparks Warnings, Shortages". The Wall Street Journal. Retrieved 26 March 2020.
- Gabler E (24 March 2020). "States Say Some Doctors Stockpile Trial Coronavirus Drugs, for Themselves". The New York Times. ISSN 0362-4331. Archived from the original on 24 March 2020. Retrieved 31 March 2020.
- Torres S. "Stop hoarding hydroxychloroquine. Many Americans, including me, need it". The Washington Post. Archived from the original on 25 March 2020. Retrieved 31 March 2020.
- "A man died after ingesting a substance he thought would protect him from coronavirus". NBC News. Retrieved 22 May 2020.
- "FDA Letter to Stakeholders: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans". U.S. Food and Drug Administration (FDA). 1 May 2020.
- Romo, Vanessa (10 April 2020). "COVID-19 Patients Given Unproven Drug In Texas Nursing Home In 'Disconcerting' Move". NPR. Retrieved 5 July 2020.
- Denise M Hinton (28 March 2020). "Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease". U.S. Food and Drug Administration (FDA). Retrieved 30 March 2020.
Having concluded that the criteria for issuance of this authorization under 564(c) of the Act are met, I am authorizing the emergency use of chloroquine phosphate and hydroxychloroquine sulfate, as described in the Scope of Authorization section of this letter (Section II) for treatment of COVID-19 when clinical trials are not available, or participation is not feasible, subject to the terms of this authorization.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Emergency Use Authorization". U.S. Food and Drug Administration (FDA). 29 March 2020. Retrieved 30 March 2020.
On March 28, 2020, FDA issued an EUA to allow hydroxychloroquine sulfate and chloroquine phosphate products donated to the Stategic National Stockpile (SNS) to be distributed and used for certain hospitalized patients with COVID-19. These drugs will be distributed from the SNS to states for doctors to prescribe to adolescent and adult patients hospitalized with COVID-19, as appropriate, when a clinical trial is not available or feasible.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Product-Specific Guidances for Chloroquine Phosphate and Hydroxychloro". U.S. Food and Drug Administration (FDA). 13 April 2020. Retrieved 13 April 2020.
- "Trump says he takes unproven hydroxychloroquine to prevent coronavirus infection". CNBC. Retrieved 25 May 2020.
- Mehra MR, Desai SS, Ruschitzka F, Patel AN (May 2020). "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis". Lancet. doi:10.1016/S0140-6736(20)31180-6. PMC 7255293. PMID 32450107. Lay summary. (Retracted, see doi:10.1016/S0140-6736(20)31324-6, )
- Mehra MR, Ruschitzka F, Patel AN (June 2020). "Retraction: "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis"". Lancet. 395 (10240): 1820. doi:10.1016/S0140-6736(20)31324-6. PMC 7274621. PMID 32511943.
- Boseley S, Davey M (4 June 2020). "Covid-19: Lancet retracts paper that halted hydroxychloroquine trials". The Guardian. Retrieved 4 June 2020.
- "Citing safety concerns, the W.H.O. paused tests of a drug Trump said he had taken". The New York Times. 26 May 2020.
- "France bans use of hydroxychloroquine, drug touted by Trump, in coronavirus patients". CBS News. 27 May 2020.
- Beaubien J (25 May 2020). "WHO Halts Hydroxychloroquine Trial Over Safety Concerns". NPR. Retrieved 27 May 2020.
- Chappell B (27 May 2020). "France Bars Use Of Hydroxychloroquine In COVID-19 Cases". NPR. Retrieved 27 May 2020.
- Risch HA (27 May 2020). "Opinion: Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis". American Journal of Epidemiology. 189 (11): 1218–1226. doi:10.1093/aje/kwaa093. PMC 7546206. PMID 32458969. S2CID 218909081.
- "COVID-19: reminder of the risks chloroquine and hydroxychloroquine". European Medicines Agency (EMA). 29 May 2020. Retrieved 29 May 2020.
- Davey M, Kirchgaessner S, Boseley S (3 June 2020). "Surgisphere: governments and WHO changed Covid-19 policy based on suspect data from tiny US company". The Guardian. Retrieved 4 June 2020.
- Horby, Peter; Landray, Martin (5 June 2020). "No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19 — RECOVERY Trial". www.recoverytrial.net. Retrieved 6 June 2020.
- "EUA Archive". U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 15 June 2020.
On June 15, 2020, based on FDA's continued review of the scientific evidence available for hydroxychloroquine sulfate (HCQ) and chloroquine phosphate (CQ) to treat COVID-19, FDA has determined that the statutory criteria for EUA as outlined in Section 564(c)(2) of the Food, Drug, and Cosmetic Act are no longer met. Specifically, FDA has determined that CQ and HCQ are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA. Additionally, in light of ongoing serious cardiac adverse events and other serious side effects, the known and potential benefits of CQ and HCQ no longer outweigh the known and potential risks for the authorized use. This warrants revocation of the EUA for HCQ and CQ for the treatment of COVID-19.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Retrieved 15 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - https://abcnews.go.com/Health/wireStory/oklahoma-seeking-return-2m-worth-anti-malaria-drug-75517793
- https://www.buzzfeednews.com/article/laurenstrapagiel/tiktoker-debunking-hydroxychloroquine-false-info
External links
| Wikiquote has quotations related to: Hydroxychloroquine |
- "Hydroxychloroquine". Drug Information Portal. U.S. National Library of Medicine.