Liquid carbon dioxide
Liquid carbon dioxide is the liquid state of carbon dioxide (CO
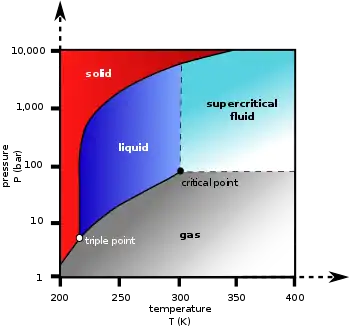
2), which cannot occur under atmospheric pressure. It can only exist at a pressure above 5.1 atm (5.2 bar; 75 psi), under 31.1 °C (temperature of critical point) and above -56.6 °C (temperature of triple point). Low-temperature carbon dioxide is commercially used in its solid form, commonly known as "dry ice". Solid CO
2 sublimes at 194.65 K (−78.5 °C; −109.3 °F) at Earth atmospheric pressure — that is, it transitions directly from solid to gas without an intermediate liquid stage. The uses and applications of liquid carbon dioxide include the extraction of virgin olive oil paste, fire extinguishers, and as a coolant.[1]
Properties

Liquid carbon dioxide is a type of liquid which is formed from highly compressed and cooled gaseous carbon dioxide. It does not form under atmospheric conditions. It only exists when the pressure is above 5.1 atm and the temperature is under 31.1 °C (temperature of critical point) and above -56.6 °C (temperature of triple point). The chemical symbol remains the same as gaseous carbon dioxide (CO
2).[2] It is transparent and odorless and the density of it is 1101 kg/m3 when the liquid is at full saturation at -37 °C.[3][4]
The solubility of water in liquid carbon dioxide is measured in a range of temperatures ranging from -29°C to 22.6 °C. At this temperature, the pressure is measured in a range from 15 to 60 atmospheres. [5]
Uses

Uses of liquid carbon dioxide include the preservation of food, in fire extinguishers, and in commercial food processes. For food preservation, liquid carbon dioxide is to refrigerate, preserve, store and soften. In a fire extinguisher, the CO
2 is stored under pressure as a liquid to act as an anti-flammable.[2] The liquid carbon dioxide not only reduces combustion by displacing oxygen, but also cools the burning surface to avoid further damage. Solvent extraction using compressed liquid CO
2 can be used in industrial processes such as removing caffeine from coffee[2] or improving the yield of olive oil production.[6]
See also
Other chemical compounds and elements are commonly used for commercial and research purposes in their liquid state:
References
- "How to Make Liquid CO2". Sciencing. Retrieved 2019-06-11.
- "What is Liquid CO2 and What Can It Be Useful For? | Co2 Gas Blog". Co2 Gas Company. 2017-10-08. Retrieved 2019-04-30.
- "Carbon dioxide", Wikipedia, 2019-04-26, retrieved 2019-04-30
- "Carbon dioxide", Wikipedia, 2019-05-20, retrieved 2019-05-21
- Stone, Hosmer W. (December 1943). "Solubility of Water in Liquid Carbon Dioxide". Industrial & Engineering Chemistry. 35 (12): 1284–1286. doi:10.1021/ie50408a015. ISSN 0019-7866.
- Romano, Raffaele; Manzo, Nadia; Montefusco, Immacolata; Romano, Annalisa; Santini, Antonello (2014-03-12). "Liquid Carbon Dioxide Use in the Extraction of Extra Virgin Olive Oil From Olive Paste". Journal of Food Research. 3 (4): 119. doi:10.5539/jfr.v3n4p119. ISSN 1927-0895.
