Neptunium(IV) oxide
Neptunium(IV) oxide, or neptunium dioxide, is a radioactive, olive green[4] cubic[5] crystalline solid with the formula NpO2. It is a common product of plutonium fission, and emits both α- and γ-particles.[3]
 | |
| Names | |
|---|---|
| IUPAC name
Neptunium(IV) oxide | |
| Other names
Neptunium oxide, Neptunium dioxide | |
| Identifiers | |
| ECHA InfoCard | 100.031.651 |
PubChem CID |
|
| Properties | |
| NpO2 | |
| Molar mass | 269 g/mol |
| Appearance | Green cubic crystals |
| Density | 11.1 g/cm3 |
| Melting point | 2,800 °C; 5,070 °F; 3,070 K[1] |
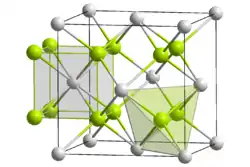
| Structure | |
| cubic crystal system, cF12 | |
| Fm3m, #225 | |
| Np, 8, cubic O, 4, tetrahedral | |
| Thermochemistry | |
Std molar entropy (S |
19.19 ± 0.1 cal·mol−1·K−1 (80.3 ± 0.4 J·mol−1·K−1)[2] |
Std enthalpy of formation (ΔfH⦵298) |
−256.7 ± 0.6 kcal·mol−1 (−1074 ± 3 kJ·mol−1)[3] |
| Related compounds | |
Other anions |
Neptunium(III) chloride Neptunium(IV) chloride |
Other cations |
Uranium(VI) oxide Plutonium(IV) oxide Promethium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Industrially, neptunium dioxide is formed from the precipitation of neptunium(IV) oxalate from a neptunium feed solution with oxalic acid, followed by calcination to neptunium dioxide. The neptunium feed solution (which includes varying oxidation states of neptunium) is reduced to a predominately neptunium(IV) solution via ascorbic acid prior to the addition of oxalic acid. A hydrazine inhibitor is initially added to the neptunium feed solution to protect the neptunium and ascorbic acid from decay.[6]
Extrapolated and balanced from "The Production of Neptunium Dioxide" by J. A. Porter[6]
Np4+ + Np5+ + Np6+ + HNO3 + C6H8O6 → 3 Np4+ + C6H6O6 + H2 + HNO3
Np4+ + C2O4H2 → Np(C2O4) • 6H2O + 2H−
Np(C2O4) • 6H2O + Δ → Np(C2O4)
Np(C2O4) + Δ → NpO2 + 2CO2
Neptunium dioxide can also be formed effectively from precipitation of neptunium(IV) peroxide, but the oxalate reduction has been found to be more industrially efficient.[6]
Purification
As a byproduct of nuclear waste, neptunium dioxide can be purified by fluorination, followed by reduction with excess calcium in the presence of iodine.[3] However, the aforementioned synthesis yields a quite pure solid, with less than 0.3% weight of impurities. Generally, further purification is unnecessary.[6]
Other properties
Neptunium dioxide contributes to the α-decay of 241Am, reducing its usual half-life an untested but appreciable amount.[7] The compound has a low specific heat capacity (900 K, compared with uranium dioxide's specific heat capacity of 1400 K), an abnormality theorized to stem from its 5f electron count.[8] Another unique trait of neptunium dioxide is its "mysterious low-temperature ordered phase". Mentioned above, it references an abnormal level of order for an actinitde dioxide complex at low temperature.[9] Further discussion of such topics could indicate useful physical trends in the actinides.
Uses
The neptunium dioxide complex is used as a means of stabilizing, and decreasing the "long term environmental burden"[10] of neptunium as a nuclear fission byproduct. Actinide-containing nuclear waste will commonly be treated so that various AnO2 (where An = U, Np, Pu, Am, etc.) complexes form. In neptunium dioxide, the neptunium is of reduced radio toxicity compared with pure neptunium metal, and is thus more desirable for storage and disposal. Neptunium dioxide has also been show to contribute to increased decay rates of radioactive metals, an application which is currently being explored.[10]
Neptunium dioxide is also used experimentally for research into nuclear chemistry and physics, and it is speculated that neptunium dioxide could be used to make efficient nuclear weapons. In nuclear reactors, neptunium dioxide can also be used as the target metal for plutonium bombardment.[10]
Furthermore, a patent for a rocket powered by neptunium dioxide is held by Shirakawa Toshihisa © 2007,[11] but there is little information available into research and production associated with such a product.
References
- Böhler, R.; M. J. Welland; F. De Bruycker; K. Boboridis; A. Janssen; R. Eloirdi; R. J. M. Konings; D. Manara (2012). "Revisiting the melting temperature of NpO2 and the challenges associated with high temperature actinide compound measurements". Journal of Applied Physics. American Institute of Physics. 111 (11): 113501. Bibcode:2012JAP...111k3501B. doi:10.1063/1.4721655.
- Westrum, Jr., Edgar F.; J. B. Hatcher; Darrell W. Osborne (March 1953). "The Entropy and Low Temperature Heat Capacity of Neptunium Dioxide". Journal of Chemical Physics. 21 (3): 419. Bibcode:1953JChPh..21..419W. doi:10.1063/1.1698923.
- Huber Jr, Elmer J.; Charles E. Holley Jr (October 1968). "Enthalpy of formation of neptunium dioxide". Journal of Chemical & Engineering Data. 13 (4): 545–546. doi:10.1021/je60039a029.
- Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill Professional. p. 271. ISBN 0-07-049439-8.
- Lide, D. R. (1998). Handbook of Chemistry and Physics 87 ed. CRC Press. p. 471. ISBN 0-8493-0594-2.
- Porter, J. A. (1964). "Production of Neptunium Dioxide". Industrial & Engineering Chemistry Process Design and Development. 4 (3): 289–292. doi:10.1021/i260012a001.
- Colle, J.-Y. (2011). "(Solid + gas) equilibrium studies for neptunium dioxide". Journal of Chemical Thermodynamics. 43 (3): 492–498. doi:10.1016/j.jct.2010.10.027.
- Serizawa, H.; Arai, Y.; Nakajima, K. (2001). "The estimation of the heat capacity of NpO2". The Journal of Chemical Thermodynamics. 33 (6): 615–628. doi:10.1006/jcht.2000.0775.
- Hotta, T. (2009). "Microscopic analysis of multipole susceptibility of actinide dioxides: A scenario of multipole ordering in AmO2". Physical Review B. 80 (2): 024408–1–024408–7. arXiv:0906.3607. Bibcode:2009PhRvB..80b4408H. doi:10.1103/PhysRevB.80.024408.
- Colle, J.-Y. (2011). "(Solid + gas) equilibrium studies for neptunium dioxide". Journal of Chemical Thermodynamics. 43 (3): 492–498. doi:10.1016/j.jct.2012.10.027.
- Toshihisa, Shirakawa. "Bibliographic data: JP2007040768 (A) - 2007-02-15". Espacenet, patent search. Retrieved 11 April 2012.