Quifenadine
Quifenadine (Russian: хифенадин, trade name: Phencarol, Фенкарол) is a 2nd generation antihistamine drug, marketed mainly in post-Soviet countries.[2][3] Chemically, it is a quinuclidine derivative.
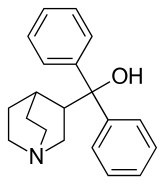 | |
| Clinical data | |
|---|---|
| Trade names | Fencarol |
| Other names | 3-Quinuclidinyldiphenylmethanol |
| Routes of administration | Oral (tablets), IM injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 45% (Tmax = 1 hour)[1] |
| Metabolism | Liver |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H23NO |
| Molar mass | 293.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The drug has antiarrhythmic properties, probably due to the presence of a quinuclidine nucleus in the molecule's core. It acts as a calcium channel blocker and influences the activity of potassium channels. In children with cardiac arrhythmia, combination therapy with quifenadone and either amiodarone or propafenone was found to be more effective than monotherapy with either amiodarone or propafenone.[4]
Indications
- Allergic rhinitis
- Acute and chronic urticaria
- Angioedema
- Dermatitis
- Atopic dermatitis
- Pruritus[1]
References
- "Fencarol (quifenadine) Tablets, for Oral Use. Full Prescribing Information". State Register of Medicines (in Russian). Retrieved 4 January 2016.
- "Quifenadine". Drugs.com. Retrieved 28 January 2014.
- Makarov L, Balykova L, Soldatova O, Komolyatova V, Serebruany V (2010). "The antiarrhythmic properties of quifenadine, H1-histamine receptor blocker in children with premature beats: a randomized controlled pilot trial". American Journal of Therapeutics. 17 (4): 396–401. doi:10.1097/MJT.0b013e3181a86987. PMID 19487925.
- Солдатова, О.Н.; Балыкова, Л.А.; Макаров, Л.М.; Комолятова, В.Н.; Чупрова, С.Н.; Солдатов, О.М.; Корнилова, Т.И. (2008-09-01). "ЭФФЕКТИВНОСТЬ ФЕНКАРОЛА В СОЧЕТАНИИ С ТРАДИЦИОННЫМИ ПРОТИВОАРИТМИЧЕСКИМИ СРЕДСТВАМИ ПРИ ЭКСТРАСИСТОЛИИ У ДЕТЕЙ". RUDN Journal of Medicine (in Russian) (3): 46–51. ISSN 2313-0245. Archived from the original on 20 November 2020. Retrieved 18 November 2020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.