Brompheniramine
Brompheniramine, sold under the brand name Dimetapp among others, is an antihistamine drug of the propylamine (alkylamine) class. It is indicated for the treatment of the symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing. It is a first-generation antihistamine and one of the drugs of highest anticholinergic activity.
 | |
| Clinical data | |
|---|---|
| Trade names | Bromfed, Dimetapp, Bromfenex, and others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682545 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 24.9 ± 9.3 hours[1] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.507 |
| Chemical and physical data | |
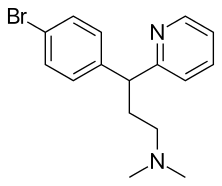
| Formula | C16H19BrN2 |
| Molar mass | 319.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It was patented in 1948 and came into medical use in 1955.[2]
Side effects
Brompheniramine's effects on the cholinergic system may include side-effects such as drowsiness, sedation, dry mouth, dry throat, blurred vision, and increased heart rate. It is listed as one of the drugs of highest anticholinergic activity in a study of anticholinergenic burden, including long-term cognitive impairment.[3]
Pharmacology
Brompheniramine works by acting as an antagonist of histamine H1 receptors. It also functions as a moderately effective anticholinergic agent, and is likely an antimuscarinic agent similar to other common antihistamines such as diphenhydramine.
Brompheniramine is metabolised by cytochrome P450s.
The halogenated alkylamine antihistamines all exhibit optic isomerism and brompheniramine products contain racaemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.
Chemistry
Brompheniramine is part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives and others including fluorpheniramine, chlorpheniramine, dexchlorpheniramine (Polaramine), triprolidine (Actifed), and iodopheniramine. The halogenated alkylamine antihistamines all exhibit optical isomerism and brompheniramine products contain racemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.
Brompheniramine is an analog of chlorpheniramine. The only difference is that the chlorine atom in the benzene ring is replaced with a bromine atom. It is also synthesized in an analogous manner.[4][5]
History
Based on this knowledge, Arvid Carlsson and his colleagues, working at the Swedish company Astra AB, were able to derive the first marketed selective serotonin reuptake inhibitor, zimelidine, from brompheniramine.[6]
Names
Brand names include Bromfed, Dimetapp, Bromfenex, Dimetane, BPN, Lodrane. It is commonly marketed as its salt brompheniramine maleate.
See also
References
- Simons FE, Frith EM, Simons KJ (December 1982). "The pharmacokinetics and antihistaminic effects of brompheniramine". The Journal of Allergy and Clinical Immunology. 70 (6): 458–64. doi:10.1016/0091-6749(82)90009-4. PMID 6128358.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 546. ISBN 9783527607495.
- Salahudeen MJ; Duffull SB; Nishtala PS; et al. (2015-03-25). "Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review". BMC Geriatrics. 15 (31): 31. doi:10.1186/s12877-015-0029-9. PMC 4377853. PMID 25879993.
- L.A. Walter, U.S. Patent 3,061,517 (1962)
- L.A. Walter, U.S. Patent 3,030,371 (1962)
- Barondes, Samuel H. (2003). Better Than Prozac. New York: Oxford University Press. pp. 39–40. ISBN 0-19-515130-5.