Stratospheric aerosol injection
The ability of stratospheric aerosols to create a global dimming effect has made them a possible candidate for use in solar radiation management climate engineering projects[1] to limit the effect and impact of climate change due to rising levels of greenhouse gases.[2] Delivery of precursor sulfide gases such as sulfuric acid,[3] hydrogen sulfide (H
2S) or sulfur dioxide (SO
2) by artillery, aircraft[4] and balloons has been proposed.[5] Non-sulfide substances such as calcite have also been proposed given their benefits to the ozone layer.[6] It appears that this could counter most changes to temperature and precipitation, take effect rapidly, have low direct implementation costs, and be reversible in its direct climatic effects.[7] However, it would do so imperfectly and other effects are possible.[8]
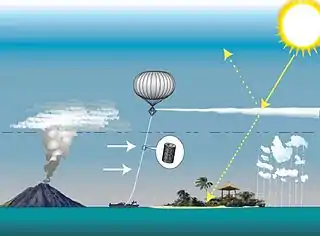
One study calculated the impact of injecting sulfate particles, or aerosols, every one to four years into the stratosphere in amounts equal to those lofted by the volcanic eruption of Mount Pinatubo in 1991,[9] but did not address the many technical and political challenges involved in potential solar radiation management (SRM) efforts.[10] If found to be economically, environmentally and technologically viable, such injections could provide a "grace period" of up to 20 years by which time atmospheric greenhouse gas pollution would need to be reduced to safe levels.
It has been suggested that the direct delivery of precursors could be achieved using sulfide gases such as dimethyl sulfide, sulfur dioxide (SO
2), carbonyl sulfide, or hydrogen sulfide (H
2S).[5] These compounds would be delivered using artillery, aircraft (such as the high-flying F-15C)[4] or balloons, and result in the formation of compounds with the sulfate anion SO42−.[5]
According to estimates, "one kilogram of well placed sulfur in the stratosphere would roughly offset the warming effect of several hundred thousand kilograms of carbon dioxide."[11]
Arguments for the technique
The arguments in favour of this approach in comparison to other possible means of solar radiation management are:
- Mimics a natural process:[12] Stratospheric sulfur aerosols are created by existing natural processes (especially volcanoes), whose impacts have been studied via observations.[13] This contrasts with other, more speculative solar radiation management techniques which do not have natural analogs (e.g., space sunshade).
- Technological feasibility: In contrast to other proposed solar radiation management techniques, such as marine cloud brightening and space sunshades, much of the required technology is pre-existing: chemical manufacturing, artillery shells, high-altitude aircraft, weather balloons, etc.[5] Unsolved technical challenges include methods to deliver the material in nanoparticular form of controlled diameter with good scattering properties.
- Scalability: Some solar radiation management techniques, such as cool roofs and ice protection, can only provide a limited intervention in the climate due to insufficient scale—one cannot reduce the temperature by more than a certain amount with each technique. Research has suggested that this technique may have a high radiative 'forcing potential'.[14] The Intergovernmental Panel on Climate Change concludes that "SAI is the most-researched SRM method, with high agreement that it could limit warming to below 1.5°C."[15]
Key side effects arguing against the technique
- Ecosystem light deprivation: Photosynthesis is the basis of life on Earth. Stratospheric aerosols reduce the level of visible light reaching land and oceans uniformly over large spatial regions. The low resolution is due to facile mixing and transport in the atmosphere.[16] Visible light, useful for photosynthesis, is reduced proportionally more than is the infrared portion of the solar spectrum due to the mechanism of Mie scattering.[17] As a result, deployment of this technology would unfortunately ensure a reduction by at least 2-5% in the growth rates of phytoplanktons, trees, and crops [18] between now and the end of the century.[19] This effect would significantly reduce human's ability to grow food and the ecosystem's ability to regenerate.
- Inhibition of solar energy technologies: Uniformly reduced net shortwave radiation would hurt solar photovoltaics by the same >2-5% as for plants because of the bandgap of silicon photovoltaics.[20] the increased scattering of collimated incoming sunlight would more drastically reduce the efficiencies (by 11% for RCP8.5) of concentrating solar thermal power for both electricity production [21] and chemical reactions, such as solar cement production.[22]
Uncertainties in efficacy and cost
It is uncertain how effective any solar radiation management technique would be, due to the difficulties modeling their impacts and the complex nature of the global climate system. Certain efficacy issues are specific to stratospheric aerosols.
- Lifespan of aerosols: Tropospheric sulfur aerosols are short lived.[23] Delivery of particles into the lower stratosphere in the arctic will typically ensure that they remain aloft only for a few weeks or months, as air in this region is predominantly descending.[24] To ensure endurance, higher-altitude delivery is needed, ensuring a typical endurance of several years by enabling injection into the rising leg of the Brewer-Dobson circulation above the tropical tropopause. Further, sizing of particles is crucial to their endurance.[25]
- Aerosol delivery: There are two proposals for how to create a stratospheric sulfate aerosol cloud, either through release of a precursor gas (SO
2) or the direct release of sulfuric acid (H
2SO
4) and these face different challenges.[26] If SO
2 gas is released it will oxidize to form H
2SO
4 and then condense to form droplets far from the injection site.[27] Releasing SO
2 would not allow control over the size of the particles that are formed but would not require a sophisticated release mechanism. Simulations suggest that as the SO
2 release rate is increased there would be diminishing returns on the cooling effect, as larger particles would be formed which have a shorter lifetime and are less effective scatterers of light.[28] If H
2SO
4 is released directly then the aerosol particles would form very quickly and in principle the particle size could be controlled although the engineering requirements for this are uncertain. Assuming a technology for direct H
2SO
4 release could be conceived and developed, it would allow control over the particle size to possibly alleviate some of the inefficiencies associated with SO
2 release.[26] - Cost: Early studies by proponents of the technique suggest that SAI might cost less than many other interventions. Costs cannot be derived in a wholly objective fashion, as pricing can only be roughly estimated at an early stage. However, some sources suggest that it would be cheap relative to cutting emissions, capturing carbon dioxide, adapting to climate impacts, or suffering climate damages.[29][30][31] The annual cost of delivering 5 million tons of an albedo enhancing aerosol to an altitude of 20 to 30 km is estimated at US$2 billion to 8 billion.[32] Around 5 million tons of SO
2 delivered annually is predicted to sufficiently offset the expected warming over the next century.[32] SO
2 can be purchased online for as little as US$500 per ton.[33] In comparison, the annual cost estimates for climate damage or emission mitigation range from US$200 billion to 2 trillion.[32]
Recent, more comprehensive research suggests that the real cost of stratospheric aerosol injection (SAI) are at least an order of magnitude higher than what its proponents suggest. A 2016 study finds the cost per 1 W/m2 of cooling to be between 5-50 billion USD/yr.[34] Because larger particles are less efficient at cooling and drop out of the sky faster, the unit-cooling cost is expected to increase over time as increased dose leads to larger, but less efficient, particles by mechanism such as coalescence and Ostwald ripening.[35] Assume RCP8.5, -5.5 W/m2 of cooling would be required by 2100 to maintain 2020 climate. At dose level required to provide this cooling, the net efficiency per mass of injected aerosols would reduce to below 50% compared to low-level deployment (below 1W/m2).[36] At a total dose of -5.5 W/m2, the cost would be between 55-550 billion USD/yr when efficiency reduction is also taken into account, bringing annual expenditure to levels comparable to other mitigation alternatives.
Other possible side effects
Climate engineering and solar radiation management in general are controversial,[37] and pose various problems and risks. However, certain problems are specific to, or more pronounced with stratospheric sulfide injection.[38] The injection of other aerosols that may be safer such as calcite has therefore been proposed.[6]
- Health effects: While the sulfate particles are natural, if any sulfate particles returned to ground level in significant amounts it would affect asthma sufferers and have other potential health effects.[39] Minimising these effects is principally achieved by ensuring the particles stay aloft as long as possible, thus reducing the tonnages returning into the lower atmosphere.
- Ozone depletion: is a potential side effect of sulfur aerosols;[40][41] and these concerns have been supported by modelling.[42] However, this may only occur if high enough quantities of aerosols drift to, or are deposited in, Polar stratospheric clouds before the levels of CFCs and other ozone destroying gases fall naturally to safe levels because stratospheric aerosols, together with the ozone destroying gases, are responsible for ozone depletion.[43]
- Whitening of the sky: Stratospheric aerosols have the potential to whiten the sky and cause more colorful sunsets, dependent on the amount being sprayed.[44] According to a study on cleaner air, the reduction of aerosol pollution has led to solar brightening in Europe and North America, which has been responsible for an increase in U.S. corn production over the past 30 years.[45]
- Tropopause warming: and the humidification of the stratosphere.[41]
- Regional warming: Based on the results of the 2014-2015 Geoengineering Model Intercomparison Project, a model with a standard stratospheric aerosol injection scenario, temperatures in the tropics would cool, and higher latitudes warm, ice sheet, and Arctic sea ice decline would still continue, albeit at a reduced rate. Extreme temperature anomalies would also still increase, but to a lesser degree. In regards to these model results, the author of the study Alan Robock noted:
If geoengineering were halted all at once, there would be rapid temperature and precipitation increases at 5–10 times the rates from gradual global warming.[46]
- Stratospheric temperature change: Aerosols can also absorb some radiation from the Sun, the Earth and the surrounding atmosphere. This changes the surrounding air temperature and could potentially impact on the stratospheric circulation, which in turn may impact the surface circulation.[47]
- Regional hydrologic responses: Based on the results of the Geoengineering Model Intercomparison Project, there would be a reduction in the global average precipitation around the world, particularly in summer monsoon regions.[46]
The injection of non-sulfide aerosols like calcite (limestone) would also have a cooling effect while counteracting ozone depletion and would be expected to reduce other side effects.[6]
Regardless of worry, such atmospheric effects are similar to medication in that amount is key, as in dose makes the poison and/or cure, furthermore, random non-artificial injections will occur anyway.
Aerosol formation
Primary aerosol formation, also known as homogeneous aerosol formation, results when gaseous SO
2 combines with oxygen and water to form aqueous sulfuric acid (H2SO4). This acidic liquid solution is in the form of a vapor and condenses onto particles of solid matter, either meteoritic in origin or from dust carried from the surface to the stratosphere. Secondary or heterogeneous aerosol formation occurs when H2SO4 vapor condenses onto existing aerosol particles. Existing aerosol particles or droplets also run into each other, creating larger particles or droplets in a process known as coagulation. Warmer atmospheric temperatures also lead to larger particles. These larger particles would be less effective at scattering sunlight because the peak light scattering is achieved by particles with a diameter of 0.3 μm.[48]
Methods
Various techniques have been proposed for delivering the aerosol precursor gases (H2S and SO
2).[2] The required altitude to enter the stratosphere is the height of the tropopause, which varies from 11 kilometres (6.8 mi/36,000 ft) at the poles to 17 kilometres (11 mi/58,000 ft) at the equator.
- Civilian aircraft including the Boeing 747-400 and Gulfstream G550/650, C-37A could be modified at relatively low cost to deliver sufficient amounts of required material according to one study,[49] but a later metastudy suggests a new aircraft would be needed but easy to develop.[50]
- Military aircraft such as the F15-C variant of the F-15 Eagle have the necessary flight ceiling, but limited payload. Military tanker aircraft such as the KC-135 Stratotanker and KC-10 Extender also have the necessary ceiling and have greater payload capacity.[4]
- Modified artillery might have the necessary capability,[51] but requires a polluting and expensive gunpowder charge to loft the payload. Railgun artillery could be a non-polluting alternative.
- High-altitude balloons can be used to lift precursor gases, in tanks, bladders or in the balloons' envelope.
Material options
Precursor gases such as sulfur dioxide and hydrogen sulfide have been considered. Use of gaseous sulfuric acid appears to reduce the problem of aerosol growth.[3] Materials such as photophoretic particles, titanium dioxide, and diamond are also under consideration.[48][52][53]
Injection system
The latitude and distribution of injection locations has been discussed by various authors. Whilst a near-equatorial injection regime will allow particles to enter the rising leg of the Brewer-Dobson circulation, several studies have concluded that a broader, and higher-latitude, injection regime will reduce injection mass flow rates and/or yield climatic benefits.[54][55] Concentration of precursor injection in a single longitude appears to be beneficial, with condensation onto existing particles reduced, giving better control of the size distribution of aerosols resulting.[56] The long residence time of carbon dioxide in the atmosphere may require a millennium-timescale commitment to SRM[57] if aggressive emissions abatement is not pursued simultaneously.
Outdoors research
Almost all work to date on stratospheric sulfate injection has been limited to modelling and laboratory work. A Russian team tested aerosol formation in the lower troposphere using helicopters.[58] The Stratospheric Particle Injection for Climate Engineering (SPICE) project planned on a limited field test in order to evaluate a potential delivery system, but this component of the project was canceled. In 2015 a group based at Harvard University described a potential field experiment, the Stratospheric Controlled Perturbation Experiment (SCoPEx), using stratospheric calcium carbonate[59] injection,[60] but the time and place have not yet been determined.[61]
Governance
Most of the existing governance of stratospheric sulfate aerosols is from that which is applicable to solar radiation management more broadly. However, some existing legal instruments would be relevant to stratospheric sulfate aerosols specifically. At the international level, the Convention on Long-Range Transboundary Air Pollution (CLRTAP Convention) obligates those countries which have ratified it to reduce their emissions of particular transboundary air pollutants. Notably, both solar radiation management and climate change (as well as greenhouse gases) could satisfy the definition of "air pollution" which the signatories commit to reduce, depending on their actual negative effects.[62] Commitments to specific values of the pollutants, including sulfates, are made through protocols to the CLRTAP Convention. Full implementation or large scale climate response field tests of stratospheric sulfate aerosols could cause countries to exceed their limits. However, because stratospheric injections would be spread across the globe instead of concentrated in a few nearby countries, and could lead to net reductions in the "air pollution" which the CLRTAP Convention is to reduce, it is uncertain how the convention's Implementation Committee and Executive Body would respond to such event.
The stratospheric injection of sulfate aerosols would cause the Vienna Convention for the Protection of the Ozone Layer to be applicable, due to their possible deleterious effects on stratospheric ozone. That treaty generally obligates its Parties to enact policies to control activities which "have or are likely to have adverse effects resulting from modification or likely modification of the ozone layer."[63] The Montreal Protocol to the Vienna Convention prohibits the production of certain ozone depleting substances, via phase outs. Sulfates are presently not among the prohibited substances.
In the United States, the Clean Air Act might give the United States Environmental Protection Agency authority to regulate stratospheric sulfate aerosols.[64]
See also
References
- Launder B. & J.M.T. Thompson (2008). "Global and Arctic climate engineering: numerical model studies". Phil. Trans. R. Soc. A. 366 (1882): 4039–4056. Bibcode:2008RSPTA.366.4039C. doi:10.1098/rsta.2008.0132. PMID 18757275.
- Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change. 77 (3–4): 211–220. Bibcode:2006ClCh...77..211C. doi:10.1007/s10584-006-9101-y.
- Pierce, J. R.; Weisenstein, D. K.; Heckendorn, P.; Peter, T.; Keith, D. W. (2010). "Efficient formation of stratospheric aerosol for climate engineering by emission of condensible vapor from aircraft". Geophysical Research Letters. 37 (18): n/a. Bibcode:2010GeoRL..3718805P. doi:10.1029/2010GL043975. S2CID 15934540.
- Robock, A.; Marquardt, A.; Kravitz, B.; Stenchikov, G. (2009). "Benefits, risks, and costs of stratospheric geoengineering". Geophysical Research Letters. 36 (19): L19703. Bibcode:2009GeoRL..3619703R. doi:10.1029/2009GL039209. hdl:10754/552099.
- Rasch, Philip J; Tilmes, Simone; Turco, Richard P; Robock, Alan; Oman, Luke; Chen, Chih-Chieh (Jack); Stenchikov, Georgiy L; Garcia, Rolando R (August 29, 2008). "An overview of geoengineering of climate using stratospheric sulphate aerosols". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 366 (1882): 4007–4037. Bibcode:2008RSPTA.366.4007R. doi:10.1098/rsta.2008.0131. PMID 18757276. S2CID 9869660.
- Keith, David W.; Weisenstein, Debra K.; Dykema, John A.; Keutsch, Frank N. (December 27, 2016). "Stratospheric solar geoengineering without ozone loss". Proceedings of the National Academy of Sciences. 113 (52): 14910–14914. Bibcode:2016PNAS..11314910K. doi:10.1073/pnas.1615572113. PMC 5206531. PMID 27956628.
- Climate Intervention: Reflecting Sunlight to Cool Earth. Washington, D.C.: National Academies Press. June 23, 2015. doi:10.17226/18988. ISBN 9780309314824.
- Daisy Dunne (March 11, 2019). "Halving global warming with solar geoengineering could 'offset tropical storm risk'". CarbonBrief. Retrieved March 14, 2019.
- Wigley, T. M. L. (October 20, 2006). "A Combined Mitigation/Geoengineering Approach to Climate Stabilization". Science. 314 (5798): 452–454. Bibcode:2006Sci...314..452W. doi:10.1126/science.1131728. PMID 16973840. S2CID 40846810.
- "Stratospheric Injections Could Help Cool Earth, Computer Model Shows – News Release". National Center for Atmospheric Research. September 14, 2006. Archived from the original on May 8, 2017. Retrieved June 15, 2011.
- David G. Victor, M. Granger Morgan, Jay Apt, John Steinbruner, and Katharine Ricke (March–April 2009). "The Geoengineering Option:A Last Resort Against Global Warming?". Geoengineering. Council on Foreign Affairs. Archived from the original on April 21, 2010. Retrieved August 19, 2009.CS1 maint: multiple names: authors list (link)
- Bates, S. S.; Lamb, B. K.; Guenther, A.; Dignon, J.; Stoiber, R. E. (1992). "Sulfur emissions to the atmosphere from natural sources". Journal of Atmospheric Chemistry. 14 (1–4): 315–337. Bibcode:1992JAtC...14..315B. doi:10.1007/BF00115242. S2CID 55497518.
- Zhao, J.; Turco, R. P.; Toon, O. B. (1995). "A model simulation of Pinatubo volcanic aerosols in the stratosphere". Journal of Geophysical Research. 100 (D4): 7315–7328. Bibcode:1995JGR...100.7315Z. doi:10.1029/94JD03325. hdl:2060/19980018652.
- Lenton, Tim; Vaughan. "Radiative forcing potential of climate geoengineering" (PDF). Retrieved February 28, 2009.
- Global warming of 1.5°C. Intergovernmental Panel on Climate Change. [Geneva, Switzerland]. p. 350. ISBN 9789291691517. OCLC 1056192590.CS1 maint: others (link)
- MacMartin, Douglas G.; Kravitz, Ben; Tilmes, Simone; Richter, Jadwiga H.; Mills, Michael J.; Lamarque, Jean-Francois; Tribbia, Joseph J.; Vitt, Francis (2017). "The climate Response to Stratospheric Aerosol Geoengineering Can Be Tailored Using Multiple Injection Locations". JGR Atmospheres. 122 (23): 12574–12590. doi:10.1002/2017JD026888.
- Erlick, Carynelisa; Frederick, John E (1998). "Effects of aerosols on the wavelength dependence of atmospheric transmission in the ultraviolet and visible 2. Continental and urban aerosols in clear skies". J. Geophys. Res. 103 (D18): 23275–23285. Bibcode:1998JGR...10323275E. doi:10.1029/98JD02119.
- Walker, David Alan (1989). "Automated measurement of leaf photosynthetic O2 evolution as a function of photon flux density". Philosophical Transactions of the Royal Society B. 323 (1216): 313–326. Bibcode:1989RSPTB.323..313W. doi:10.1098/rstb.1989.0013. Retrieved October 20, 2020.
- IPCC, Data Distribution Center. "Representative Concentration Pathways (RCPs)". Intergovernmental Panel on Climate Change. Retrieved October 20, 2020.
- Murphy, Daniel (2009). "Effect of Stratospheric Aerosols on Direct Sunlight and Implications for Concentrating Solar Power". Environ. Sci. Technol. 43 (8): 2783–2786. Bibcode:2009EnST...43.2784M. doi:10.1021/es802206b. PMID 19475950. Retrieved October 20, 2020.
- Smith, Christopher J; et al. (2017). "Impacts of Stratospheric Sulfate Geoengineering on Global Solar Photovoltaic and Concentrating Solar Power Resource". J. Appl. Meteorol. Climatol. 56 (5): 1483–1497. Bibcode:2017JApMC..56.1483S. doi:10.1175/JAMC-D-16-0298.1.
- HELIOSCSP. "Cement production with Concentrated Solar Power". helioscsp.com. Retrieved October 20, 2020.
- Monastersky, Richard (1992). "Haze clouds the greenhouse—sulfur pollution slows global warming—includes related article". Science News.
- "Breaking Christian News - Religion Headlines".
- Rasch, P. J.; Crutzen, P. J.; Coleman, D. B. (2008). "Exploring the geoengineering of climate using stratospheric sulfate aerosols: the role of particle size". Geophysical Research Letters. 35 (2): L02809. Bibcode:2008GeoRL..3502809R. doi:10.1029/2007GL032179.
- Pierce, Jeffrey R.; Weisenstein, Debra K.; Heckendorn, Patricia; Peter, Thomas; Keith, David W. (September 2010). "Efficient formation of stratospheric aerosol for climate engineering by emission of condensible vapor from aircraft". Geophysical Research Letters. 37 (18): n/a. Bibcode:2010GeoRL..3718805P. doi:10.1029/2010GL043975. S2CID 15934540.
- Niemeier, U.; Schmidt, H.; Timmreck, C. (April 2011). "The dependency of geoengineered sulfate aerosol on the emission strategy". Atmospheric Science Letters. 12 (2): 189–194. Bibcode:2011AtScL..12..189N. doi:10.1002/asl.304.
- Niemeier, U.; Timmreck, C. (2015). "ACP - Peer review - What is the limit of climate engineering by stratospheric injection of SO2?". Atmospheric Chemistry and Physics. 15 (16): 9129–9141. doi:10.5194/acp-15-9129-2015.
- Brahic, Catherine (February 25, 2009). "Hacking the planet: The only climate solution left? (NB cost data in accompanying image)". Reed Business Information Ltd. Retrieved February 28, 2009.
- "The Royal Society" (PDF). royalsociety.org. Retrieved November 18, 2015.
- Council, National Research (February 10, 2015). Climate Intervention: Reflecting Sunlight to Cool Earth. doi:10.17226/18988. ISBN 9780309314824.
- McClellan, Justin; Keith, David W; Apt, Jay (September 1, 2012). "Cost analysis of stratospheric albedo modification delivery systems". Environmental Research Letters. 7 (3): 034019. doi:10.1088/1748-9326/7/3/034019.
- "Industrial Grade 99.9% Sulfur Dioxide Gas So2 In800l Gas Cylinder - Buy Sulfur Dioxide Gas,So2 Gas,Gas Cylinder Product on Alibaba.com". www.alibaba.com. Retrieved October 1, 2020.
- Moriyama, Ryo; Sugiyama, Masahiro; Kurosawa, Atsushi; Masuda, Kooiti; Tsuzuki, Kazuhiro; Ishimoto, Yuki (2017). "The cost of stratospheric climate engineering revisited". Mitigation and Adaptation Strategies for Global Change. 22 (8): 1207–1228. doi:10.1007/s11027-016-9723-y. S2CID 157441259.
- Heckendorn, P; Weisenstein, D; Fueglistaler, S; Luo, B P; Rozanov, E; Schraner, M; Thomason, M; Peter, T (2009). "The impact of geoengineering aerosols on stratospheric temperature and ozone". Environ. Res. Lett. 4 (4): 045108. Bibcode:2009ERL.....4d5108H. doi:10.1088/1748-9326/4/4/045108.
- Niemeier, U.; Timmreck, U. (2015). "What is the limit of climate engineering by stratospheric injection of SO2". Atmos. Chem. Phys. 15 (16): 9129–9141. Bibcode:2015ACP....15.9129N. doi:10.5194/acp-15-9129-2015.
- Sigal Samuel (March 13, 2019). "The case for spraying (just enough) chemicals into the sky to fight climate change; A new study says geoengineering could cut global warming in half — with no bad side effects". Vox.com. Retrieved March 14, 2019.
- Robock, A. (2008). "20 reasons why geoengineering may be a bad idea" (PDF). Bulletin of the Atomic Scientists. 64 (2): 14–19. Bibcode:2008BuAtS..64b..14R. doi:10.2968/064002006. S2CID 145468054.
- "Archived copy". Archived from the original on May 4, 2011. Retrieved November 24, 2017.CS1 maint: archived copy as title (link)
- Tabazadeh, A.; Drdla, K.; Schoeberl, M. R.; Hamill, P.; Toon, O. B. (February 19, 2002). "Arctic 'ozone hole' in a cold volcanic stratosphere". Proceedings of the National Academy of Sciences. 99 (5): 2609–2612. Bibcode:2002PNAS...99.2609T. doi:10.1073/pnas.052518199. PMC 122395. PMID 11854461.
- Kenzelmann, Patricia; Weissenstein, D; Peter, T; Luo, B; Fueglistaler, S; Rozanov, E; Thomason, L (February 1, 2009). "Geo-engineering side effects: Heating the tropical tropopause by sedimenting sulphur aerosol?". IOP Conference Series: Earth and Environmental Science. 6 (45): 452017. Bibcode:2009E&ES....6S2017K. doi:10.1088/1755-1307/6/45/452017.
- Heckendorn, P; Weisenstein, D; Fueglistaler, S; Luo, B P; Rozanov, E; Schraner, M; Thomason, L W; Peter, T (2009). "The impact of geoengineering aerosols on stratospheric temperature and ozone". Environmental Research Letters. 4 (4): 045108. Bibcode:2009ERL.....4d5108H. doi:10.1088/1748-9326/4/4/045108.
- Hargreaves, Ben (2010). "Protecting the Planet". Professional Engineering. 23 (19): 18–22.
- Olson, D. W., R. L. Doescher, and M. S. Olson (February 2004). "When the Sky Ran Red: The Story Behind The Scream". Sky & Telescope. 107 (2): 29–35. Bibcode:2004S&T...107b..28O.CS1 maint: multiple names: authors list (link)
- "A bright sun today? It's down to the atmosphere". The Guardian. 2017.
- Robock, Alan (2014). "Stratospheric Aerosol Geoengineering". Geoengineering of the Climate System. pp. 162–185. doi:10.1039/9781782621225-00162.
- Ferraro, A. J., Highwood, E. J., Charlton-Perez, A. J. (2011). "Stratospheric heating by geoengineering aerosols". Geophysical Research Letters. 37 (24): L24706. Bibcode:2011GeoRL..3824706F. doi:10.1029/2011GL049761. hdl:10871/16215.CS1 maint: multiple names: authors list (link)
- Keith, D. W. (September 7, 2010). "Photophoretic levitation of engineered aerosols for geoengineering". Proceedings of the National Academy of Sciences. 107 (38): 16428–16431. Bibcode:2010PNAS..10716428K. doi:10.1073/pnas.1009519107. PMC 2944714. PMID 20823254.
- McClellan, Justin; Keith, David; Apt, Jay (August 30, 2012). "Cost Analysis of Stratospheric Albedo Modification Delivery Systems". Environmental Research Letters. 7 (3): 3 in 1–8. doi:10.1088/1748-9326/7/3/034019.
- Smith, Wake; Wagner, Gernot (2018). "Stratospheric aerosol injection tactics and costs in the first 15 years of deployment". Environmental Research Letters. 13 (12): 124001. Bibcode:2018ERL....13l4001S. doi:10.1088/1748-9326/aae98d.
- PICATINNY ARSENAL DOVER N J. "PARAMETRIC STUDIES ON USE OF BOOSTED ARTILLERY PROJECTILES FOR HIGH ALTITUDE RESEARCH PROBES, PROJECT HARP". Archived from the original on January 14, 2017. Retrieved February 25, 2009.
- Keith, D.W. and D. K. Weisenstein (2015). "Solar geoengineering using solid aerosol in the stratosphere". Atmos. Chem. Phys. Discuss. 15 (8): 11799–11851. Bibcode:2015ACPD...1511799W. doi:10.5194/acpd-15-11799-2015.
- Ferraro, A. J.; Charlton-Perez, A. J.; Highwood, E. J. (January 27, 2015). "Stratospheric dynamics and midlatitude jets under geoengineering with space mirrors and sulfate and titania aerosols" (PDF). Journal of Geophysical Research: Atmospheres. 120 (2): 414–429. Bibcode:2015JGRD..120..414F. doi:10.1002/2014JD022734. hdl:10871/16214.
- English, J. M.; Toon, O. B.; Mills, M. J. (2012). "Microphysical simulations of sulfur burdens from stratospheric sulfur geoengineering". Atmospheric Chemistry and Physics. 12 (10): 4775–4793. Bibcode:2012ACP....12.4775E. doi:10.5194/acp-12-4775-2012.
- MacCracken, M. C.; Shin, H. -J.; Caldeira, K.; Ban-Weiss, G. A. (2012). "Climate response to imposed solar radiation reductions in high latitudes". Earth System Dynamics Discussions. 3 (2): 715–757. Bibcode:2012ESDD....3..715M. doi:10.5194/esdd-3-715-2012.
- Niemeier, U.; Schmidt, H.; Timmreck, C. (2011). "The dependency of geoengineered sulfate aerosol on the emission strategy". Atmospheric Science Letters. 12 (2): 189–194. Bibcode:2011AtScL..12..189N. doi:10.1002/asl.304.
- Brovkin, V.; Petoukhov, V.; Claussen, M.; Bauer, E.; Archer, D.; Jaeger, C. (2008). "Geoengineering climate by stratospheric sulfur injections: Earth system vulnerability to technological failure". Climatic Change. 92 (3–4): 243–259. doi:10.1007/s10584-008-9490-1.
- Izrael, Yuri; et al. (2009). "Field studies of a geo-engineering method of maintaining a modern climate with aerosol particles". Russian Meteorology and Hydrology. 34 (10): 635–638. doi:10.3103/S106837390910001X. S2CID 129327083.
- Adler, Nils (October 20, 2020). "10 million snowblowers? Last-ditch ideas to save the Arctic ice". The Guardian. ISSN 0261-3077. Retrieved October 27, 2020.
- Dykema, John A.; et al. (2014). "Stratospheric controlled perturbation experiment: a small-scale experiment to improve understanding of the risks of solar geoengineering". Phil. Trans. R. Soc. A. 372 (2013): 20140059. Bibcode:2014RSPTA.37240059D. doi:10.1098/rsta.2014.0059. PMC 4240955. PMID 25404681.
- "SCoPEx Science". projects.iq.harvard.edu. Retrieved October 27, 2020.
- Convention on Long-Range Transboundary Air Pollution art. 1, Nov. 13, 1979, 1302 U.N.T.S. 219, Article 1
- Vienna Convention for the Protection of the Ozone Layer, opened for signature Mar. 22, 1985, 1513 U.N.T.S. 293, Article 1
- Hester, Tracy D. (2011). "Remaking the World to Save It: Applying U.S. Environmental Laws to Climate Engineering Projects". Ecology Law Quarterly. 38 (4): 851–901. JSTOR 24115125. SSRN 1755203.
Further reading
- Crutzen, P. J. (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change. 77 (3–4): 211–220. Bibcode:2006ClCh...77..211C. doi:10.1007/s10584-006-9101-y.
- Keutsch Research Group, Harvard University. "Stratospheric Controlled Perturbation Experiment (SCoPEx)". Harvard.edu. Retrieved March 14, 2019.
External links
- What can we do about climate change?, Oceanography magazine
- Global Warming and Ice Ages: Prospects for Physics-Based Modulation of Global Change, Lawrence Livermore National Laboratory
- The Geoengineering Option:A Last Resort Against Global Warming?, Council on Foreign Relations
- Geo-Engineering Climate Change with Sulfate Aerosols, Pacific Northwest National Laboratory
- Geo-Engineering Research, Parliamentary Office of Science and Technology
- Geo-engineering Options for Mitigating Climate Change, Department of Energy and Climate Change
- Unilateral Geoengineering, Council on Foreign Relations
- Rasch, Philip J; Tilmes, Simone; Turco, Richard P; Robock, Alan; Oman, Luke; Chen, Chih-Chieh (Jack); Stenchikov, Georgiy L; Garcia, Rolando R (November 13, 2008). "An overview of geoengineering of climate using stratospheric sulphate aerosols". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 366 (1882): 4007–4037. Bibcode:2008RSPTA.366.4007R. doi:10.1098/rsta.2008.0131. PMID 18757276. S2CID 9869660.
- US 5003186 "Stratospheric Welsbach seeding for reduction of global warming"
- As planet warms, scientists explore 'far out' ways to reduce atmospheric CO2 on YouTube PBS NewsHour published on March 27, 2019 animation of SCoPEx