Tawny owl
The tawny owl or brown owl (Strix aluco) is a stocky, medium-sized owl commonly found in woodlands across much of the Palearctic. The tawny owl is a member of the genus Strix, that is also the origin of the family's name under Linnaean taxonomy.[3] Its underparts are pale with dark streaks, and the upperparts are either rufous, brown or grey. Several of the eight recognised subspecies have each of the main colour variations.[4][5] This nocturnal bird of prey hunts a wide variety of prey species, but usually primarily takes small mammals such as rodents. Tawny owls usually hunt by dropping from a perch to seize their prey, which they typically swallow whole. In more urban areas, its diet includes a higher proportion of birds, while in arid subtropics many invertebrates such as insects are taken.[6][7][8] Other important prey can include frogs with other vertebrate prey taken fairly rarely.[9] Vision and well-developed hearing adaptations combined with silent flight aid its night hunting.[10] The tawny owl is capable of catching smaller owls, but is itself vulnerable to larger raptors, like eagle-owls or goshawks.[6][11] This species typically nests in a tree hollow, wherein they are likely to gain protection of their eggs and young against potential predators.[6] The tawny owl is non-migratory and highly territorial. Many young birds starve if they cannot find a vacant territory once parental care ceases.[7][12] Although many people believe this owl has exceptional night vision, its retina is no more sensitive than a human's and its asymmetrically placed ears are key to its hunting by giving it excellent directional hearing. Its nocturnal habits and eerie, easily imitated call, have led to a mythical association of the tawny owl with bad luck and death.[13]
| Tawny owl | |
|---|---|
.jpg.webp) | |
| A grey morph individual | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Strigiformes |
| Family: | Strigidae |
| Genus: | Strix |
| Species: | S. aluco |
| Binomial name | |
| Strix aluco | |
 | |
| Synonyms[2] | |
| |
Description
Form and colouring

This is a robust owl that is quite distinct for its large, rounded head. Tawny owls have no ear tufts but do possess a prominent facial disc rimmed in slightly dusky feathers.[4] Despite having a broad facial disc rim, the facial disc is largely indistinct from the surrounding feathers in markings and colour, unlike some other owls that have relative bold facial disc patterns.[10] The eyes are blackish-brown rimmed (at times imperceptibly) narrowly by the pale fleshy edges of the blue-grey eyelids.[4] The underparts of all morphs are whitish in base colour.[14] The underside feathers are barred in a dusky colour with several crossbars, producing a herringbone pattern.[4] Their typical rich brownish colour often camouflages it well against a variety of woodland types.[10] Tawny owls are spotted with white along the line of the scapulars, forming a white spotted "shoulder".[10] The tail is rather short and the wings are broad. The tarsi and toes are densely feathered. Tawny owls possess relatively thick and heavy legs and feet and the talons are rather powerful and quite decurved.[6][4] In flight they can appear fairly big and broad, large headed and rounded on the wings.[10] The tawny owl often flies with long glides on rounded wings, less undulating and with fewer wingbeats than other Eurasian owls, and typically at a greater height. The flight of the tawny owl is relatively heavy and slow, particularly at takeoff.[7] They can appear to be a heavy flier but are capable of surprising maneuverability within woods, flying with utter silence.[10] As with most owls, its flight is silent because of its feathers' soft, furry upper surfaces and a fringe on the leading edge of the outer primaries.[15][16] Annual moult is usually complete in tawny owls but not all wing feathers are moulted each year.[7] Feathers are moulted gradually between June and December.[17] Of 91 males and 214 females in Great Britain, 17-19% did not moult any primaries, while 1-6% replaced all primaries, about 6% of males and 2% of females annually replaced median-primaries, while about 11% of males and 4% of females replaced annually their median secondaries.[18] Young individuals can sometimes be diagnosed to age roughly by the state of wing moult. However, some variation in wing moult accounts for mistaken identification by age of 3 year old owls for younger ages due to the fact that they retain some worn juvenile wing feathers. Moult tends to occur after young fledge in late summer-early autumn for mature owls.[19]

Plumage coloring in this species can be very variable. The nominate race in particular has two main morphs which differ in their plumage colour. The predominant morphs are grey and rufous, with a more minor intermediate or brown morph also sometimes occurring in various races; sometimes each morph may intergrade.[4] The plumage colour is genetically controlled. There is some indication that pleiotropy has led to the colouring diversity in the species.[20] Studies, largely based in Italy and in Finland, based on contour feathers indicate that grey morph owls are more densely insulated and better suited to surviving cooler conditions, which is roughly in keeping with the respective morph distribution. Meanwhile, in warmer and wetter and/or more humid conditions, rufous morph individuals are better adapted.[20] However, similar studies on climate, habitat and colour morph found no strong correlation between colour morph, habitat and survivorship in Switzerland.[7] Studies on colour morphs also indicated that higher levels of melanin, such as darker rufous morphs, may suffer higher rates of parasitism, body mass loss through the season across all ages but on the contrary also had higher growth rates for nestlings and were more likely to breed every year than grey morphs in Italy and Switzerland regardless of prey resources than grey morphs.[7][20] Studies in Finland indicate that grey morph tawny owls have more reproductive success, better immune resistance, and fewer parasites than other morphs. The data on grey morphs having the aforementioned advantages is also supported in Italian data.[20][21][22] Although this might suggest that eventually the darker morphs could disappear, the owls show no colour preference when choosing a mate, so the selection pressure in favour of the grey morph is reduced. There are also environmental factors involved. The Italian study showed that brown-morph birds were found in denser woodland, and in Finland, Gloger's rule would suggest that paler birds would in any case predominate in the colder climate.[23][24] In Poland, neither primary morph was necessary predominant, with 51.4% of 107 owls being rufous morph and 46.7% being grey morphs and this may qualify as a transitional zone.[25] Other areas studied for colour morph proportions showed the following, in England (sample size 31): 55% rufous 39% grey and 6% intermediate; in France (315): 65% rufous and 35% grey; in Spain (54): 26% rufous, 65% grey and 9% intermediate; in Germany (50): 10% rufous and 90% grey; in Czech Republic (102): 32.3% rufous, 61.8% grey and 5.9% intermediate; Switzerland (79): 33% rufous and 67% grey.[7]
Size

The tawny owl is a medium-sized species of owl. This species is sexually dimorphic; the female is notably larger than the male, often averaging up to 5% longer and can average more than 25% heavier.[26] This is sometimes called reverse sexual dimorphism (RSD) as it runs opposite of most birds, wherein males are usually larger, but almost all unrelated groups of birds of prey display some degree of RSD.[27] Of European owls, the tawny owl ranks as fourth most dimorphic by weight and fifth most dimorphic by wing dimensions. The prevalent hypothesis is that RSD occurs in birds of prey due to the considerable rigors of the breeding cycle.[28] Of the Strix in the Northern Hemisphere it is perhaps the smallest.[4] Total length in the tawny owl ranges from 36 to 46 cm (14 to 18 in).[4][29] Average length in Denmark was found to be 36.7 cm (14.4 in) in 10 males and 37.7 cm (14.8 in) in 18 females.[30] Average total length in Spain was 38.9 cm (15.3 in) in 10 males and 39.3 cm (15.5 in) in 12 females.[31] Wingspan may vary from 81 to 105 cm (32 to 41 in).[32][33] In Denmark, the average wingspan in 9 males was 89.7 cm (35.3 in) and in 12 females was 91.9 cm (36.2 in) and in Spain the average was 87 cm (34 in) in 14 males and 88.7 cm (34.9 in) in 12 females.[30][31] Among standard measurements, across the various subspecies, the wing chord of males can range from 248 to 323 mm (9.8 to 12.7 in) whilst that of the female may vary from 255 to 343 mm (10.0 to 13.5 in). Tail length can vary from 148 to 210 mm (5.8 to 8.3 in) while the less widely measured linear variants of total bill length have been reported at 28 to 35 mm (1.1 to 1.4 in) and tarsus at 45 to 63 mm (1.8 to 2.5 in).[5][7] Voous claimed a mean weight of 474 g (1.045 lb) for males and 583 g (1.285 lb), which would render them 70% more massive than an average long-eared owl (Asio otus) and 60% more massive than an average western barn owl (Tyto alba) despite these species' similar appearance by size.[6][34] In Denmark, males and females were found to vary in weight from 392 to 692 g (0.864 to 1.526 lb), with an average weight across seasons was 490 g (1.08 lb) for both sexes, or 440 g (16 oz) for males and 539.7 g (1.190 lb) for females. In Danish owls, weights were lowest during brooding and fledgling stages and highest in winter, varying up to 12% and 10%, respectively, in males and females.[30] Weight fluctuations by season were even more pronounced in France, where the mean weights of males and females in winter and late spring differed by 17% in males and nearly 20% in females.[35] In southern Finland body mass were studied instead by age, with the division of 3 years considered for 172 females and 135 males. In the males, weight was nearly the same across the age divide, being 481.6 g (1.062 lb) in the younger males and 480.2 g (1.059 lb) in the older ones, however older females were notably larger than young ones, with the younger females averaging 689.1 g (1.519 lb) and older ones averaging 731.6 g (1.613 lb). Older females in the Finnish study were found on average to also breed earlier, be more productive and adapt better to varying prey conditions.[36] In England and Scotland, the weights of freshly dead owls was studied against live ones weighed in the wild, with 79 dead females averaging 484.5 g (1.068 lb) against an average of 533 g (1.175 lb) for 22 live females. Meanwhile, in males 384.1 g (13.55 oz) was found to be the average for 63 dead individuals, while 20 live ones averaged 408.6 g (14.41 oz). It was found in English and Scottish tawny owls that the weight down to which males and females could survive starvation could go as low as 325 g (11.5 oz) in males and 390 g (14 oz) in females.[37] In Spain, the average weight of 16 males was 406.2 g (14.33 oz) and for 19 females was 460 g (1.01 lb).[31] In total, weight can vary from 304 to 800 g (0.670 to 1.764 lb) in full grown tawny owls.[7][37]
.jpg.webp)
Hearing and auditory morphology
Hearing is important for a nocturnal bird of prey, and as with other owls, the tawny owl's two ear openings differ in structure and are asymmetrically placed to improve directional hearing. A passage through the skull links the eardrums, and small differences in the time of arrival of a sound at each ear enables its source to be pinpointed. The left ear opening is higher on the head than the larger right ear and tilts downward, improving sensitivity to sounds from below.[38] While the species does show the typical ear asymmetry of an owl and the right ear is consistently larger, the average differences of 7-13% are relatively modest for an owl.[6][39] Both ear openings are hidden under the facial disk feathers, which are structurally specialized to be transparent to sound, and are supported by a movable fold of skin (the pre-aural flap).[6] The ear slits average reportedly 21 to 23 mm (0.83 to 0.91 in) on the left and 22.5 to 26 mm (0.89 to 1.02 in) on the right.[40][39] The movable pre-aural skin flap on tawny owl averages 9.5 mm (0.37 in) on the left and 10.5 mm (0.41 in) on the right.[40][39] The tawny owls has a comparable ear morphology to the Ural owl (Strix uralensis). They tend to have a less complicated ear structure than those of Asio species but a more complicated, well-developed and relatively larger ear structure than those of other large generas of typical owl like the Bubo genus or Otus genus.[6][10][39] The internal structure of the ear, which has large numbers of auditory neurons, gives an improved ability to detect low-frequency sounds at a distance, which could include rustling made by prey moving in vegetation.[6] The tawny owl's hearing may be ten times better than a human's,[6] and it can hunt using this sense alone in the dark of a woodland on an overcast night. However, the patter of raindrops can make it likely difficult for these owls to detect faint sounds, and prolonged wet weather, especially the crashing din of heavy rain, can lead to starvation if the owl cannot hunt effectively.[10][38] The tawny's range is estimated to average 0.4-0.7 kHz with a maximum of around 3 kHz.[10][40] The maximum range, in comparison, is up to 6 kHz in the long-eared owl and to 1 kHz in the eagle owl.[6][41]
Vocalizations
Advertising calls and most threat and supplanting calls are mostly by males while both sexes may engage in contact calls and alarm calls.[6] Autumn boundary disputes may occur with excited varied wails and screams between hoots (or "caterwauling").[6] The male has a quavering advertising song hoo...ho, ho, hoo-hoo-hoo-hoo or whooooh uk whooooook. It is described as "a clear, fluted, long-drawn hoot with a wailing quality".[4] Broken down, the male's song is considered as about three notes drawn together into one, often with an upward inflection and emphasis on the middle note, followed after a brief pause followed by a very short ho, uk or hu and continuing after a further short interval with a long tremolo of staccato notes, often rising or falling slightly in pitch and drawn out in the end. On average, the male's song is about 17 seconds in duration.[6][4][42] The song may carry up to 1.5 to 2 km (0.93 to 1.24 mi) to human perception.[43] More than 99% of the time, it was found that individual males could be distinguished via spectrogram in Italy.[42] A female territorial call is somewhat like the male's but is hoarser, less clear and somewhat higher in pitch, transcribed as cher oooOOooo followed by chro cher-oooOOooo cooEEooooo.[4][43] William Shakespeare used this owl's calls in Love's Labour's Lost (Act 5, Scene 2) as "Then nightly sings the staring owl, Tu-whit; Tu-who, a merry note, While greasy Joan doth keel the pot", but this stereotypical call is actually a duet, with the female making the kew-wick contact call.[4][14] The male's response to the females kewick contact call is more varied, sometimes muffled and fluting notes, sometimes wavering or crooning notes and sometimes a more dissimilar hissing chruuuuuu.[44][45][46] The calls of tawny owls are easily imitated by blowing into cupped hands through slightly parted thumbs, and a study in Cambridgeshire found that this mimicry produced a response from the owl within 30 minutes in 94% of trials.[47] Recordings of various calls may also be an effective way for researchers to study territories and owl responsiveness. English male tawny owls were responsive both male and female calls, the latter perhaps due to an interest for mates, while females usually only responded to recordings of female calls.[48]
.jpg.webp)
Song posts are often only 250 to 300 m (820 to 980 ft) from their roost sites.[44][45] In an Italian study, 12 males responded much more strongly to recordings of "stranger" male tawnys than to recordings of neighboring male owls known to them, in some cases coming to physically attack the recorder when the "stranger" call was playing.[49] A study within Spain recording only spontaneous vocalizations that only a low percentage of territories could be detected this way, about 12%, and that males spontaneously called about 2 to 4 times more frequently than females.[50] A male's response to a broadcast song appears to be indicative of his health and vigour; owls with higher blood parasite loads use fewer high frequencies and a more limited range of frequencies in their responses to an apparent intruder.[51] In Italy, males hooted more emphatically when the female was in the vicinity.[52] The vocal activity of tawny owls depends on sex, annual cycle stage and weather, with males being more vocal than females year-round, with peak vocal activity during courtship, late winter to early spring, and post-breeding in early autumn, the two times of year when territories are most hotly contested. The least frequent vocalizing as a whole being is in December–February and during mid-May to early September, but most especially June to July.[10][50] Males in particular may call even at the quietest times of year, i.e. usually when he is presumably excited or irritated.[46][43] Female territorial hooting is almost entirely confined to autumn.[7] In Italy, females were more aggressive on average than males in response to playback despite lower response levels and exhibited much higher aggression when both members of the pair were present.[53] A correlation was also made in the amount of singing relative to habitat, with owls with territories in farmland responding more vigorously to imitated calls than those in woodland.[47][54] More moonlit nights showed more aggressive vocal displays in Pavia, Italy despite other seasonal and temporal factors seeming to play no role.[52] In France, studied tawny owls vocalized significantly less during rainy nights, with a very strong 8 fold difference in discrimination threshold (carrying 614 to 74 m (2,014 to 243 ft)) and 69 fold difference in audible broadcast area (118.4 to 1.7 ha (292.6 to 4.2 acres)) in dry against rainy weather.[55] In 50 British tawny owls that were studied in terms of singing duration and quality no correlation was made between breeding success and amount of singing, though larger males sang less but with more emphasis on the last note while those with more evidence of parasites sang more on average.[56] A study of the two main European races, one based in Italy (S. a. aluco) and one in England (S. a. sylvatica), showed the primary song of the male differed significantly in five of the 13 parameters considered, duration of the second note, lowest frequency of the first note, and frequency modulation amplitude being the most important variables and the two races could be aurally discriminated with a high success rate (86.7%). Variation was also found within the races by habitat especially. A patchwork pattern of possibly culturally transmitted hoot variations was therefore recognized, which may indicate tawny owls have dialects.[57] The calls of the subspecies in southeastern Europe and the Caucasus (S. a. willkonskii) similarly differed in half of the six parameters considered from the nominate subspecies, with the singing having an overall lower tone in keeping with the race's slightly larger size.[58]
Further described calls by tawny owls include piercing coo-wik or cu-weeehl cries, apparently expressing aggression. When disturbed at the nest, these owls may utter a series of yelping uett-uett-uett.. notes.[4] Boundary disputes frequently cause males to utter a wett wett, weck weck weck or gweck gweck gweck call, while females do a significantly less deep-sounding version of this. Females may also engage in a similar vocalization before launching a protective attack against predators.[46] A further bubbling call, uttered by both sexes, the males' version being softer and lower, the females being harsher, liken to human uttering ooo sound while moving tongue back and forth, due to the soft rolling sound, but also has been compared to the drumming sound of a common snipe (Gallinago gallinago). Although fairly frequent during high intensity pair interactions (such as during nest inspections), the bubbling call is difficult to detect except at close range so is probably underreported.[43][59][60] A pig-like grunting has sometimes been reported as emitted by males during courtship displays.[61] Leading up to food deliveries by the male, the female may utter a food excitement call, kiv-kiv-kiv-kiv..., culminating in a peeping whistling sii-sii-si-siiiii as she receives prey.[43] Other calls uttered by females witt-witt (before copulation) and rapid kikikikiiii (during copulation), both of which may be the same call, while a high-pitched, trilling sound similar to European green toad (Bufo virdis), sometimes transcribed as lee-lee-lee, is uttered by the female in similar contexts.[7][43][62] The female may call hung-hung or ung-ung-ung-haug-haug when comforting their nestlings or trying to get reluctant young to eat.[63][64] A distraction display call may be engaged in by either parent, wavering iiiii or keeee, similar to the chipping of a passerine, has been described as "unsteady flute-like piping of despair".[7] Males may utter a chochochocho, apparently to express gentleness when in close quarters with its mate.[7][43] A mysterious call described as the long call is of unknown meaning, long call, consisting of a moaning, elongated note of unknown meaning, often isolated from any other kind of vocalization and reminiscent of the mewing of a herring gull (Larus argentatus), keeeee keeuuuh keeuhkuhkuh.[7] Other mysterious calls recorded for tawny owls have included tooting, chittering, crowing, screeching or mewing, as well as soft, plaintive squeaks by females.[7] The young owls in nest beg for food with a drawn-out cheek or cheheee, sziii-szi, psji-ii or tsjuk.[4][7] Delicate, piping pipipipi calls may uttered by nestlings in discomfort (often recorded when the mother interrupts brooding).[46] From when they can actively feed themselves to when they are fledged, the young draw out their call to a wheezy, louder and more boisterous tsi-weep, being less highed and squeaky than the begging call of the long-eared owl.[10] By the first year, the young tawny owls have an adult voice but it is usually slightly higher pitched.[7]
Vision
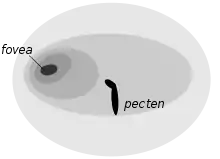
An owl's eyes are placed at the front of the head and have a field overlap of 50–70%, giving it better binocular vision than diurnal birds of prey (overlap 30–50%).[38][66] The diameter of the eye reaches about 16 to 23 mm (0.63 to 0.91 in), against 11 mm (0.43 in) in the long-eared owl, while the tawny's axial length reaches 29 to 35.7 mm (1.14 to 1.41 in).[6][66] The tawny owl's retina has about 56,000 light-sensitive rod cells per square millimetre (36 million per square inch); although earlier claims that it could see in the infrared part of the spectrum have been dismissed,[67] it is still often said to have eyesight 10 to 100 times better than humans in low-light conditions. However, the experimental basis for this claim is probably inaccurate by at least a factor of 10.[9] The owl's actual visual acuity is only slightly greater than that of humans, and any increased sensitivity is due to optical factors rather than to greater retinal sensitivity; both humans and owl have reached the limit of resolution for the retinas of terrestrial vertebrates.[68][69][70][71] In addition to middling visual acuity relative to other vertebrates, the colour discrimination in the vision of this owl may be limited.[72][73]

Adaptations to night vision include the large size of the eye, its tubular shape, large numbers of closely packed retinal rods, and an absence of cone cells, since rod cells have superior light sensitivity. There are few coloured oil drops, which would reduce the light intensity.[74] Unlike diurnal birds of prey, owls normally have only one fovea, and that is poorly developed except in daytime and dusk hunters like the short-eared owl (Asio flammeus).[38] When compared to a diurnal bird like a rock dove (Columba livia), the tawny owl's ability to navigate at night does not appear to lie in its light gather power or number of rod receptors but instead is due to the differences in the retinal neural integration mechanisms which is only possible due to the absolutely large size of the rental image only because of its absolutely large sized retinal image.[75][76] It was hypothesized that the tawny owls ability to navigate their surroundings may be correlated in part due to familiarity with the environment of their territory.[77]
Confusion species

Generally speaking, the tawny owl is unlikely to be mistaken for other owls.[78] Its size, squat shape and broad wings distinguish it from other owls found within its range; other Strix and eagle-owls can be somewhat similar in shape, but are much larger.[7] Despite two other European species being included in the Strix genus, the tawny is rather distinct from the others.[4] The Ural owl is notably larger with proportionately smaller dark brown eyes, a proportionately smaller head and the underparts are streaked without crossbars. Furthermore, Ural owls have a proportionately longer and more distinctly barred tail.[4][79][80] The great grey owl (Strix nebulosa) is much larger than a tawny owl with a huge head, a proportionately longer tail, a more uniform dark-greyish plumage, a darker facial disc with distinct concentric lines and relatively smaller yellow eyes.[4] The desert or Hume's owls (Strix hadorami) (as well as its sister species, the Omani owl (Strix butleri)) are almost entirely allopatric in distribution from tawny owls. Both species range into some mutual areas, such as the northern Middle East including Israel but almost always in different areas and habitats. The Hume's owl is smaller than the tawny owl and more sandy-colored with orange-yellow to pale ochraceous-orange eyes.[4][32] Among owls of the Asio genus, all species are smaller to a certain degree and quite different looking. All Asio species also prefer more open habitats than tawny owls as well.[6][4] Marsh owl (Asio capensis) which overlaps scarcely in Northwestern Africa has smaller ear tufts and is rather uniform earthen-brown above with pale facial disc. Short-eared owl (Asio flammeus) has smaller ear tufts, yellow eyes and streaked underparts. The long-eared owl (Asio otus) is slighter in build and weight than a tawny owl with prominent ear tufts, orange eyes and different plumage patterning.[6][4][80] All eagle-owls and fish-owls are much larger than tawny owls with prominent ear tufts and distinct plumages.[4]
Taxonomy and subspecies
The species was first described by Carl Linnaeus in his landmark 1758 10th edition of Systema Naturae under its current scientific name.[81] The binomial derives from Greek strix "owl" and Italian allocco, "tawny owl" (from Latin ulucus "screech-owl").[26] Some early descriptions upon review were found to have somehow conflated the very different barn owl with the scientific name Strix aluco, which in turn engendered some confusion.[82]

The tawny owl is a member of the wood-owl genus Strix, part of the typical owl family Strigidae, which contains all species of owl other than the barn owls. Conservatively, about 18 species are currently represented in this Strix genus, typically being medium to large sized owls, characteristically round-headed and lacking ear tufts, which acclimate to living in forested parts of various climatic zones.[33][83] Four owls native to the neotropics are sometimes additionally included with the Strix genus though some authors include these in a separate but related genus, Ciccaba.[84][85] The tawny owl is smallish for a Strix species, the smallest of the circumpolar species, mildly larger than the species from the neotropics and tropical species from Africa and the Middle East, and slightly to considerably smaller than the Asian tropical species.[5][33] Strix owls have an extensive fossil record and have long been widely distributed.[86] The genetic relationship of true owls is somewhat muddled and different genetic testings has variously indicated that Strix owls are related to disparate appearing genera like Pulsatrix, Bubo and Asio.[4][5][85][87] Tropical species, such as the mottled owl (Strix virgata) and the African wood owl (Strix woodfordii), the latter once considered a close relative to the tawny owl, morphological differ from and have smaller outer ear areas than tawny owls.[6][88]
The tawny owl is thought to be a close relative of the Ural owl. Authors have hypothesized that the origin of the two species divided following Pleistocene continental glaciations segregated a southwest or southern group in temperate forest (i.e. the tawny) from an eastern one inhabiting cold, boreal ranges (i.e. the Ural). After retreat of the continental ice masses, the ranges more recently penetrated each other.[6][40][89] While the life history details of the tawny and Ural owls are largely corresponding, nonetheless the species have a number of morphological differences and are largely adapted to different climates, times of activity and habitats.[6][61] Based on Strix fossil species from Middle Pleistocene (given the name Strix intermedia) in variously the Czech Republic, Austria and Hungary show from leg and wing bones indicate an owl of intermediate form and size between Ural and tawny owls.[90][91][92][93] However, fossils of a larger and differently proportioned Strix owl than a tawny owl, identified as Strix brevis, from Germany and Hungary from before the Pleistocene (i.e. Piacenzian) suggest a more complicated evolutionary and distributional history.[6][91][92][93][94] A hybrid was recorded in captivity between a male Ural and a female tawny owl, which managed to produce two offspring that were intermediate in size and had a more complex song that was also shared some characteristics with both species' vocalizations.[95]
A number of owls that were considered conspecific with the tawny owl are now widely considered to be separate species via conclusive genetic studies. These consist of the desert or Hume's owl and its sister species, the recently separated and range-restricted Omani owl.[96][97][98] Another species even more recently found to be distinct from tawny owls is the little-known Himalayan owl (Strix nivicolum).[99][100] Yet another former subspecies, the Maghreb owl (Strix mauritanica), was recently split from this species on the grounds of genetics via phylogeography, with this subdesert owl differing from the tawny in its cooler colour tone and differing calls.[101] In all four separated species there is no evidence that the tawny owl breeds in the same areas as them, making each species allopatric, though the desert and tawny's range nearly abuts in some parts of the Middle East such as northern Israel.[97][102] Also, in the Western Himalayas both the tawny and Himalayan owls are known to occur but there is likely a gap of several hundred kilometers in distribution with tawny mostly restricted to the Pakistani side while the Himalayan is rarely found west of Himachal Pradesh.[1][33][103] Furthermore, the desert and Omani species pair and the Himalayan species are considerably different based on superficial appearance (far more so than true tawny owl subspecies), have distinct voices and appear to have slightly different nesting habits than tawny owls.[32]
Subspecies
The tawny owl subspecies are often poorly differentiated, and may be at a flexible stage of subspecies formation with features related to the ambient temperature, the colour tone of the local habitat, and the size of available prey. Consequently, various authors have historically described between 10 and 15 subspecies.[6] The total number of subspecies was once considered as totaling at 11 subspecies but is now reduced due to the separation of the Himalayan owl and its own further two subspecies down to about eight subspecies.[4][5][32] The currently recognised subspecies are listed below.[104]
| Subspecies | Range | Described by (parentheses indicate originally in a different genus) | Description |
|---|---|---|---|
| S. a. aluco | North & Central Europe from southern Scandinavia to the Mediterranean and Black Sea and European Russia | Linnaeus, 1758 | Markedly polymorphic with all three morphs known. Some brown morph individuals bear indistinct concentric lines on the facial disc and tend to have a disc rim is dark brown.[4] Generally, birds of the nominate subspecies are rather pale below with sparser markings and more creamy base colour showing than other European tawny owls.[7] Study of genetic phylogeography of showed that the population of the nominate race in the Balkans originated as a postglacial occupation of northern territories, although these populations do interbreed with populations of two other clines, in the Alps and Pyrenees.[105] This is a medium-sized subspecies. In wing chord males may measure 259 to 286 mm (10.2 to 11.3 in) and females may measure 268 to 298 mm (10.6 to 11.7 in).[4][5] The tail measures 148 to 166 mm (5.8 to 6.5 in) in males and 154 to 171 mm (6.1 to 6.7 in) in females. In both sexes, the tarsus may measure 45 to 53 mm (1.8 to 2.1 in) and the bill 28.5 to 34.5 mm (1.12 to 1.36 in).[4][5] Unlike the species overall, the nominate subspecies neatly corresponds to Bergmann's rule (wherein animal are larger farther from the Equator). In northern Italy, the average wing chord in males and females, respectively, was 267 and 274.5 mm (10.51 and 10.81 in) and body mass averaged 445 and 543 g (0.981 and 1.197 lb) in the two sexes.[7] Much farther north in Finland, nominate race owls were notably larger, averaging 275 and 287 mm (10.8 and 11.3 in) in wing chord and 480 and 699 g (1.058 and 1.541 lb) in body mass.[4][36] Taken as a whole, the nominate includes both the heavier and lightest recorded birds in the tawny owl species.[5][7][36] |
| S. a. sylvatica | West Europe including Great Britain and the Iberian Peninsula | Shaw, 1809 | Generally the appearance of S. a. sylvatica is not dissimilar from the nominate subspecies but on average it is more boldly patterned with considerably less white base colour showing below, particularly with a richer average hue in rufous and intermediate morph individuals.[4][7] More significantly, the main song of this subspecies differs slightly from that of nominate subspecies based on spectrograms.[57] Linearly, this is a fairly small subspecies, averaging around 10% smaller than S. a. aluco, and may include the smallest known tawny owls going on standard measurements.[4][80] However, average weights do not significantly differ from those of other subspecies with published weights.[7][80] Wing chord measurements may range from 248 to 280 mm (9.8 to 11.0 in) in males and from 255 to 296 mm (10.0 to 11.7 in) in females.[4][5][31][35] In Spain, the tail could measure 140 to 191 mm (5.5 to 7.5 in), averaging 167.8 mm (6.61 in), the tarsus could measure 47 to 61 mm (1.9 to 2.4 in), averaging 53.85 mm (2.120 in) and the bill could measure 24 to 31 mm (0.94 to 1.22 in), averaging 28.5 mm (1.12 in).[31] Average wing chord in males from England and France were 260.9 and 268 mm (10.27 and 10.55 in) respectively while those of females were 273.6 and 276 mm (10.77 and 10.87 in).[37][35] Average weights in England and France were 408.6 and 427.8 g (14.41 and 15.09 oz) for 22 and 66 males and 533 and 567 g (1.175 and 1.250 lb) in 20 and 50 females.[37][35] Live adult weights can range from 335 to 580 g (0.739 to 1.279 lb) in males and 430 to 780 g (0.95 to 1.72 lb).[35] |
| S. a. biddulphi | NW India and Pakistan | Scully, 1881[106] | This isolated subspecies is fairly distinct for its stark grey morph, with other morphs either rare or non-existent.[4] It has a more stark apparent whitish base colour apparent with a strong grey wash on the head and mantle as well as strong herringbone patterning below. Altogether, it lacks the warmer tones common in more westerly tawny owls and its colouring is not dissimilar from a Ural owl but for the herringbone pattern.[4][107][108] Although at times apparently hypothesized as a separate form,[4] most authors continue to retain it as a proper subspecies of tawny owl.[5][7] Another distinct feature of S. a. biddulphi is its relatively large size and it appears to be the largest bodied race of tawny owl, although published weights are not known.[5] Wing chord in males was found to be 285 to 323 mm (11.2 to 12.7 in) whilst that of females measures 320 to 345 mm (12.6 to 13.6 in). The tail may measure 191 to 210 mm (7.5 to 8.3 in) while a single bird had a tarsal length of 51 mm (2.0 in) and two birds had bill lengths of 33 and 35 mm (1.3 and 1.4 in).[4][5] |
| S. a. willkonskii | Palestine, Asia Minor to N Iran and the Caucasus up to southeastern Europe | (Menzbier, 1896) | In likelihood, this subspecies includes the formerly described race of S. a. obscurata.[4] On the whole, this race tends to be somewhat more richly coloured than the nominate subspecies. Particularly unique within this subspecies is a dark morph which is somewhat rufous but can grade into an almost coffee brown hue.[4][32] Although some authors consider this a small subspecies,[4] measurements suggest it is more so of intermediate size.[5] In fact, average sizes may be exceed those of nominate race tawny owls from further north in Europe and the male song may consequently have a slightly deeper tone as well.[58] Wing chord in males may measure 255 to 296 mm (10.0 to 11.7 in) while females may measure 282 to 305 mm (11.1 to 12.0 in). Furthermore, weight of one male S. a. willkonskii was 510 g (1.12 lb) while one female weighed 582 g (1.283 lb).[5] |
| S. a. sanctinicolai | W Iran, NE Iraq | (Zarudny, 1905)[109] | This little known subspecies is apparently a rather pale and washed-out form, as excepted for a species that lives in subdesert region.[4] Although the only known measurements obtained have been of wing chord it appears to be one of the smaller forms of the tawny owl. Males may measure from 255 to 273 mm (10.0 to 10.7 in) and females have been known to measure 270 to 285 mm (10.6 to 11.2 in).[5] |
| S. a. harmsi | The area once known as Turkestan, which today includes portions of six various countries. | (Zarudny, 1911)[110] | This is a relatively dark hued form, which may be in some way intermediate with the Himalayan owl based on colouring but is still considered part of the tawny owl species.[5][32] This race is quite large based on wing chord dimensions, and may rival S. a. biddulphi as the largest form of tawny owl. Measurements for males are 303 to 316 mm (11.9 to 12.4 in) while females they are 318 to 332 mm (12.5 to 13.1 in).[4][5] |
| S. a. siberiae | Central Russia from the Urals to about the Irtysh river in Western Siberia | Dementiev, 1933 | This race is paler still than the nominate race with a large amount of dazzling white apparent on the sparsely marked underside, which tends to bare relatively few crossbars.[111] This is a relatively large subspecies, being fairly similar in size to the nominate birds from Scandinavia.[6] This race is up to 12% larger than Central European nominate birds.[6][112] Wing chord may measure from 280 to 300 mm (11 to 12 in) in males and from 301 to 311 mm (11.9 to 12.2 in) in females. A single owl measured 175 mm (6.9 in) in tail length and 33 mm (1.3 in) in bill length.[5] Unexpectedly, the reported weights for S. a. siberiae are not high relative to most reported in Europe and come in at a similar range as those reported for linearly rather smaller populations such as S. a. sylvatica in France.[5][35] Reported body mass for S. a. siberiae is 450 to 490 g (0.99 to 1.08 lb) in males and 590 to 680 g (1.30 to 1.50 lb) in females.[5] |
Distribution and habitat
This species is found throughout much of Iberian Peninsula, though spotty distribution here, with the largest gap where absent being in southeastern Spain (where still not completely absent).[1][113] The tawny owl is also found throughout England and Scotland, but is not present in some of less well wooded areas of northern Scotland.[10] Their range is almost continuously from throughout France to eastern Europe within mainland Europe and continuously from Estonia, Latvia and Denmark in the north down through most of Italy (including northern Sicily).[1][80][79][114] Tawny owls may be absent to rare in some swaths of southeastern Europe such as smallish parts of Bosnia and Herzegovina, Montenegro, central Bulgaria and southern Romania where the habitat probably becomes too mountainous and is similarly absent in the mountainous parts of Switzerland and northernmost Italy. In Scandinavia, the tawny owl ranges through much of southern and central Norway (where they probably reach their northern limit as a species in central Nordland), southern Sweden (up to Dalarna and southeastern Norrland) and southern Finland (jogging up slightly farther north along the coast of the Gulf of Bothnia).[1][80] Their occurrence in Finland is quite recent, with the species estimated to have colonized the country independently around the year 1878, and, possibly in sync with warming temperatures, tawny owls have expanded their range in other relatively northern countries like Norway, the Netherlands and Belgium.[6][115] The tawny owl is considered a rare vagrant to the Balearic and Canary Islands.[116] Also, the tawny owl ranges throughout coastal (to the coast of the Mediterranean and the Black Sea) and central Turkey, most of Georgia and Azerbaijan, Lebanon, far western Syria, northernmost Israel, northeastern Jordan, northern and southeastern Iraq and western, northern and central Iran.[1][114][117] After a wide gap of distribution, the range reassumes in central and eastern Uzbekistan, southern Kazakhstan, northern Tajikistan, northern Kyrgyzstan and northeasternmost China (i.e. the area once consider Turkestan). After another gap, the range resumes in northeasternmost Afghanistan, northern Pakistan, eastern Tajikistan and north India (mostly western Jammu and Kashmir).[1][6][118][119] The tawny owl is also distributed in a large portion of Russia, though mainly the southwestern part, ranging up as far north up to about the city of Petrozavodsk in the west, Lake Tolvayarvi and Kama River in the central part with the range stopping at roughly the Irtysh river in western Siberia thence more or less continuously from there down into northwestern Kazakhstan.[1][111] Records of the species expanding their range along the Irtysh and far the west up into Karelia may show that the species is expanding its range north much as it is in Europe.[120]
Habitat

The preferred habitat of the tawny owl is temperate deciduous forest and mixed forest with some access to clearings. They too may habituate to riverine forests, parks, large gardens with old trees, open landscapes with wooded patches and avenues of trees in open agriculture.[4] The species prefers "richly structured habitat" with old, mature trees available.[121] Since they naturally tend to utilize tree hollows as nesting sites, sections of forest or woods with available snags may be ideal.[7] They tend to occupy pure coniferous forest only near edges or when clearings and glades exist. Often areas in the conifer forest, especially the taiga in the north, where the tawnys will occur show a mixture of some deciduous tree growth such as birches and poplars.[6] In the taiga-dominant environments of vast Russia, tawny owls are usually restricted to broadleaf stands often in river drainages, parks, orchards and cultivated lands, often where woods of Quercus, Tilia and Betula stand with plentiful broken snags and dead trees.[111] Locally, the tawny owl has been known to be adaptive to subalpine forest dominated by conifers, such as the pine forests in the Spanish ranges of Sierra de Gredos and Sierra de Guadarrama.[122] Similarly, in southern Poland, they reported occur in spruce-fir dominated forests.[123] Also the species can habituate to rocky areas as long as they have scattered trees and bushes from which to execute hunting.[4] Locally, tawny owls are quite adaptive to living near or in human settlements, extending to towns or cities, most often within timbered gardens or tree-line pavement areas. They have adapted to living in parks or wooded suburban fringes of almost every major European city, including London and Berlin.[4][7] They also live in and around even larger cities just outside of Europe, such as Istanbul and Moscow.[124][125] Although tawny owls occur in urban environments, they are less likely to occur at sites with high noise levels at night.[126] While this owl can settle in very young forest as long as nest boxes are available, woods with trees too young to support typical hunting behaviours from a prominent perch may be suboptimal.[6] In Lithuania, it was found that nest boxes would booster the population in openings of the forest, interiors of mature forest and even grassland but no increase was noted in young forest in a state of recovery.[127] In the well-studied population of Monks Wood, England, those living in more continuous sections of the woods (stands exceeding 4 ha (9.9 acres)) had more territorial skirmishes and overlapping territories while within farmland parts would be clustered around available wooded stands. In the Monks Woods, intermediate woods were probably preferable with less direct competition and more food was likely.[128][129] In a Romanian study, tawny owls were rare in glades within the forest where substantial gaps occurred and were clustered around very old stands of trees, possibly being restricted from the more prey-rich glades by interspecific competition.[130] In central Italy, 560 territories were studied in various habitats such as urban parks, mesophilic woods, sclerophyllous woods, and mountainous beech woods, with the most attractive and highest density type being in sclerophyllous woods and lowest in urban woods and mountainous beech.[131] Generally, tawny owls occur in lowland areas but also may occur in mountainous areas (i.e. not exceeding 550 m (1,800 ft) in Scotland).[132] They generally do not exceed 1,600 to 1,800 m (5,200 to 5,900 ft) above sea level in the Alps but may live at up to 2,650 m (8,690 ft) on Piz Lagrev in Switzerland.[133][134] Tawny owls may live at elevations of over 2,000 m (6,600 ft) in parts of Armenia, Turkey and Tien Shen.[111] The species may even occur at elevations of up to 4,200 m (13,800 ft) in the Himalayas.[4]

Behaviour
.jpg.webp)
The tawny owl is generally quite nocturnal, but are sometimes briefly active during daylight. This is usually the case when young have to be fed and male owls may need to be active continuously for up to 11 hours in order to obtain enough prey.[6][4] In a probably exceptional event, some tawny owls were observed to mix with a flock of black-headed gulls (Chroicocephalus ridibundus) in extracting earthworms on a plowed field in England in broad daylight.[135] Of the three European owls in the Strix genus, the tawny is by far the least prone to be active during daylight.[136] Nocturnal activity by tawny owls starts on average 18–22 minutes earlier and ends on average 10 minutes later than that of nearby long-eared owls.[137] Radio study in Monks Woods, revealed that upon nightfall, males nesting in continuous woodland spent 40% less time flying, covering an average distance of 74.9 m (246 ft) per hour, than those nesting in farmland, which covered an average of 148 m (486 ft) per hour. The males would perch for about 8 minutes on average.[138] These owls may roost by day amongst dense foliage, quite often on a branch close to the trunk, or in a natural hole in a tree or rock formation, in a hole or crevice of a wall.[4] They at times will make use of manmade perches in suburban areas, such as utility poles, peaked roofs, chimney pots, tall fences, billboards or television antenna by dusk, while during the day they often tuck away in hollies, evergreens, oaks and/or thick ivy.[6][10] On occasion, they may found roosting even in the attics of large buildings, barns or sheds, inside church towers or the chimneys of houses.[4] One may be able to locate tawny owls by looking for whitewash but, unlike long-eared owls, tawny owls changes perch sites with some regularity so they tend to be less detectable overall.[10] Often finding tawny owls during daylight is done by listening for noisy mobbing of a discovered owl by other birds, especially by large and/or bold passerines, or by squirrels during the day. Usually, the often fairly drowsy owls are unable to counterattack or kill their wary tormentors and may at times depart and try to seek out another roost.[10][139] A radiotelemetry study of 22 owls in Denmark researched the effect perch use has in mitigating potential mobbings or predation acts. It was found that juveniles were more likely to use to secluded, hidden roosts whereas adults with hatched young through independent young were more likely to perch in the open apparently to protect their offspring. Adults were more likely to perch in open and closer to the ground when prey supplies were lower than were they were not.[140] Though this is generally a quite cold-hardy species, a study near the northern limits of the species range in central Norway showed that due to thermoregulation that the owls locally had to compensate for the climate by conserving energy via incremental feeding activity.[141]
Territoriality and movements

The tawny owl is a highly territorial owl that seldom leaves its home range. Tawny owls maintain territories through the signature male and female hooting songs.[6] Although they tend to most vigorously defend their territories in autumn, when year-old birds may try to supplant either member of a pair (though often unsuccessfully), and least so when actively incubating and brooding in spring and early summer, these owls can easily be provoked to defend their territory at any time of the year.[6][4][7][80] Not infrequently, territorial fights become heated, potentially drawing all members of two pairs and/or escalating into a potentially fatal physical confrontations, and may be embellished with bill-snapping and wing raising.[6][44][142] During male territorial displays, after giving chase, the initial male is often chased right back, occasionally seesawing as such multiple times, occasionally hitting branches or wrestling one another to the ground.[45][46] Occasional fights with long-eared owls along territorial edges are recorded too.[43] In September–December in Wytham Woods, 0.42 boundary disputes were recorded per hour in woods and 0.14 per hour at night in farmland, most occurred when pairs were within 3 m (9.8 ft) of each other.[44] Territories tend to be markedly stable over time, in some pairs at Wytham Woods, territorial lines have been roughly the same over a 2 decade period.[7] Single pairs have been known maintained territories for up to 10 years in Russia and even up to 13 year in the Berlin area.[46][111] In Gribskov, Denmark, the overlapping mutual range of both members of a pair averaged 82% in summer and 56% in winter, while on average 9% of the home range overlapped with neighboring pairs.[143] Habitat appears to be key in territory size, i.e. in English farmland (Warwickshire) (10) territories were inversely related to the amount of closed woodland they contained, whereas in fragmented woodland (Cambridgeshire) (23) territories were dependent strongly on the size of "core" woodland with owls in smaller or more isolated stands having larger territories.[128] In Wytham Woods, territories average around 7.3 ha (18 acres) on sparse limestone ground, but are 13.8 ha (34 acres) on average where dense ground cover grows over clay earth.[44] Subsequently, in British deciduous forests it was estimated that the average territory would be around 18.2 ha (45 acres) in deciduous woods, 37.4 ha (92 acres) in mixed farmland and 46.1 ha (114 acres) in spruce stands.[44][144] In farmland areas of Aberdeenshire, Scotland, the defended border of the territory was up to 3 km (1.9 mi), with 17-40% of the territories were used exclusively for hunting.[145] Spanish studies, in Bizkaia, show a fairly low density of around 0.72 territories per square kilometer for 1704 occupied territories found in area of 2,348 km2 (907 sq mi).[146] Lower still densities were found in Murcia far to the south in Spain, where the tawny owls must nest on rock formations, with 17 territories were found per 100 km2 (39 sq mi).[147]
In Central Europe, in prime areas, territories are often about 25 to 35 ha (62 to 86 acres), seldom to 50 ha (120 acres), and have a defended boundary of about 2 to 3 km (1.2 to 1.9 mi).[7] Study blocks of western Germany held 42 territories on 50 km2 (19 sq mi) and 21 territories in 25 km2 (9.7 sq mi).[148] Elsewhere in central Europe, in Kozłowiecki forest of Poland, pair occupancy increased from 2.4 to 4.6 per 10 km2 (3.9 sq mi) between 1991 and 2006, due to conserving of forest, i.e. trees with cavities, and increasingly mild weather.[149] The Polish city of Warsaw was found to hold 1.2-1.6 pairs per 10 km2 (3.9 sq mi) in the city and 0.8-1 pairs per 10 km2 (3.9 sq mi) in the general area, with 40-60 pairs found in Warsaw metropolitan.[150] In the Polish area of Lubin, territory sizes averaged 18.8 ha (46 acres) with much variation based on pair density and season, with the smallest territories down to 10.8 ha (27 acres) in summer and the largest in autumn at 30.9 ha (76 acres).[151] In the city centre of Pavia in Italy, the average number of pairs per square kilometer was 0.9-1.1 or an average territory size of 17.9 ha (44 acres), while in the rural areas nearby the average territory size was 22 ha (54 acres). Higher densities still were detected in the nearby Po plains.[12][152]
In a study of 586 territories on 22 study plots in central Italy, average territory size in peak thermophilous woods was 7.1 ha (18 acres) while other woodland types from urban parks to montane beech habitats ranged in average size from 10.8 to 22.4 ha (27 to 55 acres).[153][154] Territory size varied in the area of Rome by habitat, with wooded city parks, with 3.3 territories per square kilometer, and well-wooded suburbs, with 5.7 territories per square kilometer, holding peak numbers among the habitat types and having an average territory size of 17.6 ha (43 acres), while developed areas of the city, rural areas and farmland (where average territory was 183.4 ha (453 acres)) all held considerably lower densities of territories.[155][156] A few recorded territories in Denmark were found to be 27 to 50 ha (67 to 124 acres) in size.[43] Two studies in Belgium placed territory size at 65 to 75 ha (160 to 190 acres), elsewhere it was estimated in Belgium that there was 1 pair per 72 ha (180 acres).[157][158] In Montenegro's montane Bjelasica area, 6.1 territories were registered per 10 km2 (3.9 sq mi).[159] In Moldova, the average density of territories was 8.3 per 10 km2 (3.9 sq mi).[160] In Sweden, larger territories are necessary, i.e. 2 mature females over 89 and 146 ha (220 and 360 acres).[161] A similarly very large range was reported for tawny owls in the Trondheim area of Norway.[162]
The tawny owl is a not a migratory bird and adults tend to be highly residential, maintaining their home range and territory throughout the year. However, juvenile dispersal can occur over dozens to rarely hundreds of kilometres.[6] In southern Finland, juveniles rarely move more than 100 km (62 mi) away from their nest of origin, doing so in multidirectional movements.[6][7] In Sweden, most movements by juveniles are to less than 50 km (31 mi) away from their nest of origin, rarely more so. Exceptionally a juvenile tawny was recorded to cover 745 km (463 mi) northwesterly from Västergötland to Västerbotten.[163] The record movement recorded for a tawny owl from Scandinavia (or possibly from anywhere) apparently is 745 km (463 mi).[4] Further south in Europe, long-distance movements tend to be much rarer, and perhaps such movements are rarer than any other owl.[6][133] Rare records show movements of up to 270 to 450 km (170 to 280 mi) movements from the northern reaches of central Europe.[6][133]
Dietary biology
%252C_kand%C5%BEe%253B_Tawny_Owl_claws.jpg.webp)
The tawny owl is an opportunistic and generalized predator. Peak hunting activity tends to occur largely between dusk to midnight, with owls often following an erratic hunting pattern, perhaps to sites where previous hunts were successful.[7][138] When feeding young, hunting may need to be prolonged into daylight in the early morning.[45][15][164] Based on hand-reared young owls that re-released into the wild, hunting behaviour is quite innate rather than learned.[165] Normally this owl hunts from a perch.[4][7] Perching bouts usually last from about 8 to 14 minutes depending largely on habitat.[138] Tawny owl's hunting from a perch or pole can recall a buzzard and the two take similar prey sizes as well. However, high initial speed and maneuvering among trees and bushes with great dexterity may allow it to surprise relatively large prey, more like a goshawk.[6] The tawny owl is capable of lifting and carrying off in flight individual prey weighing up to at least 320 g (11 oz).[166] Their middle talon, the most enlarged claw on owls, measures an average of 19.1 mm (0.75 in). While not as large as those of the Ural owl, the talons are extremely sharp, stout and quite decurved. The claws are considered to be visibly more overdeveloped than those of other European mid-sized owls and the footspan including the claws is fairly larger as well, at an average of about 13.4 cm (5.3 in).[6][31][80] The hunting owl often extends its wings to balance and control prey upon impact.[167] Alternatively, this species may hunt from flight. This occurs from 2 to 3 m (6.6 to 9.8 ft) over the ground, often over open habitats such as bushes, marsh or grassland, forming a quartering or zigzag pattern over the opening. During these flights they cover about 30 to 50 m (98 to 164 ft) before changing direction.[168] Hunting from flight was surprisingly prevalent in a Swedish study of two radio-tagged birds, with 34% of study time spent hunting from flight while 40% of the study time was spent on hunting from a perch.[168] In a similar study in England, less than 1% of time was spent hunting from flight.[138] In a more deliberate variation of hunting from flight, the hunting owl may examine crags and nest boxes or also hover around prey roosts. In the latter type of hunts, the tawny owls may strike branches and/or beat their wings together in front of denser foliage, bushes or conifers in order to disturb and flush prey such as small birds and bats, or may dive directly into said foliage.[6][167][169][170] Hovering has also been recorded in differing circumstances, including one incidence of an owl hunting a small bird that was caught on the wing after a hovering flight.[171][172] Tawny owls have also taken bats on the wing as well (such as ones snatched from near streep lamps when attempting to hunt themselves) and have been seen to hawk large, relatively slow-flying insects such as some beetles and moths in flight.[6][169] Caterpillars may too be taken from trees.[169] Usually these hunting variations are correlated with poor weather hampering the capture of preferred prey.[7][173] Tawny owls eat worms with relative frequency, as they often hear them apparently from below the surface and snatch them up from shallow dirt or below leaf litter. Their worm-hunting style recalls worm hunting techniques by most other birds and they were recorded to eat 0.39 worms per minute during an hour of observation in England and were sometimes seen to feed on worms during daylight.[6][135][174][175] Other hunting from the ground has been observed, often of insects such as beetles, but tawny owls have also been reported to "leap" upon from a ground vantage point in order to capture a vole, quite like foxes often do.[6][176][177] There are now many accounts of tawny owls feeding on carrion from a wide range of sources, including hares, rats, sheep, and trout.[178][179][180][181]
).jpg.webp)
Upon capture, small prey like shrews and rodents are often swallowed whole, while others may be torn into pieces. Often prey is dismembered in order to more easily ingest it whole, i.e. decapitating mice, removing the legs from frogs while birds like sparrows are also regularly decapitated (with the head often eaten separately) and nearly all avian prey is plucked before being consumed.[6][182][183][184] One tawny owl was observed to eat a squirrel by leaving the head intact and peeling the skin back from the neck, apparently leaving bones in place while consuming the flesh.[185] Indigestible items, including fur, feathers, bones (which sometimes visibly protrude out of the peller), sometimes intestines and invertebrate carapaces, are regurgitated in large pellets, that can be anywhere in typical size from 20.3 to 67 mm (0.80 to 2.64 in) long with a diameter of 17 to 30 mm (0.67 to 1.18 in). The pellets are typically grey coloured and are found in groups under trees used for roosting or nesting. At least some tawny owl pellets can measure up to 84 mm (3.3 in) long and can include large objects such as an intact 10 cm (3.9 in) bill of a snipe.[6][7][183][186] Undigested material coughed up often reveals different prey than pellets.[6] Estimated daily food requirements for a tawny owl is 73.5 g (2.59 oz), which is proportionately lower (at about 14% of their own body mass) than the estimates for other medium-sized owls in Europe (at 23-26% of their own body mass), therefore tawny owls can appear to live off of relatively little food quite efficiently.[187]
Prey spectrum
The tawny owl takes an extremely wide range of prey species. The global prey spectrum for tawny owls includes well over 400 prey species.[6][7][188] They generally prefer small mammals in their diet, especially various species of rodent, where they are available.[188] However, they are one are the least specialized owls in Europe when it comes to prey selection and can broadly be described as extremely opportunistic.[6][80] Tawny owls respond to access of prey concentrations of virtually any variety, including birds, amphibians and insects as well as sometimes reptiles and fish, by taking them in large numbers, sometimes equal or even (more infrequently) greater numbers than mammalian prey.[7][188][189] The difference between the generalist tawny owl and a specialized rodent-hunter like the long-eared owl was illustrated in a semi-captive experience where the two owl species were exposed to different classes of wild prey as they encountered it. In this experiment, only small mammals and roosting sparrows were attacked and eaten by both, though flying sparrrows were avoided by long-eared owls and not by the tawnys. In the stated study, the tawny owls would kill and eat amphibians and fish, while the long-eared owls would rarely kill and never eat these types of prey.[190] In a study of five European biomes, with about 45 prey species per biome, the tawny owl was estimated to have tied for the second most prey species per biome after the Eurasian eagle-owl (Bubo bubo).[191] Another European study found the mean food niche breadth, i.e. the estimated average by number of prey species per nest or study site, the tawny owl surpassed all European owls within the two of the three main regions of non-British Europe, with 5.84 mean food niche breadth in central Europe and 4.3 food niche breadth in the Mediterranean region. In the latter study, the eagle-owl food niche breadth was listed as 2.4 and 3.3 in these regions, respectively (tawny owls were excluded from analysis in the Scandivanian region due to their marginal range there).[192] The tawny owl mostly focuses on fairly small-sized prey. One estimation of the mean prey size taken in all of Europe for the tawny owl was 34.5 g (1.22 oz). In northern and central Europe, older studies place the mean prey size taken as usually between 29 and 40 g (1.0 and 1.4 oz).[6][80][169] Another study, of the aforementioned 5 European biomes, showed a drastically lower mean estimated prey size of 18.6 g (0.66 oz), even slightly lower than the mean prey size taken by an owl like the Eurasian pygmy owl (Glaucidium passerinum), which weighs about one-eighth as much as tawny owl.[191] Individual dietary studies show that the mean prey mass taken by tawny owls can vary from 12.6 to 130 g (0.44 to 4.59 oz) depending on prey access.[6][191][193] A central Italian study showed how habitat type and resulting prey composition can vary mean prey size considerably, with broadleaf highland forest having a mean prey mass of 26.6 g (0.94 oz), mixed forest having a mean mass of 37.7 g (1.33 oz), urban areas having a mean prey mass of 40.3 g (1.42 oz) and coppice woodland having a high mean prey mass of 73.1 g (2.58 oz).[194]
Mammals

Tawny owls will potentially take any small mammals that they encounter. This was illustrated in Poland where the number of species taken by the owls was greater than the number of species biologists could capture themselves.[195] They primarily take and derive most of their food energy from rodent prey.[7] Dietary staples in much of their range are in particular the long-tailed Apodemus, commonly called field or wood mice, and the short-tailed rodents known as voles. Amongst voles, the widely distributed bank vole (Myodes glareolus) tends to be the most favored type over large portions of the range, though different species of the Microtus genus can become locally rather prominent.[80][188] Microtus voles tend to forage in more open habitats such as fields than do the wooded edge-favoring bank vole and especially Apodemus mice, and so are usually selected more so where the preferred rodent types are rare or absent.[6] Previous studies claimed that Apodemus mice were preferred where available over bank voles as the latter was considered "somewhat diurnal",[6] however bank voles like many voles are more correctly considered cathemeral, potentially active any time of day or night, and may actually be readily accessible to hunting tawny owls.[196][197] However, the bank voles favoring of heavier ground cover does limit access to them in the warmer months, whereas Apodemus mice are more likely to continue forage on open ground adjacent to woods and tend to be preferred at this time.[198] It was found that bank voles become more vulnerable to tawny owls in areas where enlarged deer herds consume more of the ground cover.[199] A broad study of different nations within central Europe found that Apodemus mice and bank voles could alternately take the primary food mantle, and that the variation of which was favorite was likely due to differing habitat and forest composition characteristics in the given regions.[200]
In Finland, bank and Microtus voles were taken more or less in equivalent occurrence to their observed populations in the field.[201] Similarly, in Poland, they took yellow-necked mice (Apodemus flavicollis) adults roughly in proportion to their occurrence in the wild. On the other hand, per the Polish study, juvenile yellow-necks were taken much less and subadults much more than their occurrence in the wild. Here, the tawny owls took slightly larger specimens on average than the average recorded in wild, at an estimated mean size taken of 34 g (1.2 oz).[202] In a study from Denmark, yellow-necked mice and bank voles that were caught by tawny owls were disproportionately large, adult males (55% and 73% of the time, respectively).[203] In central Lithuania, tawny and long-eared owls took common voles (Microtus arvalis) than were 24% heavier on average than those encountered in the wild, which averaged 16.45 g (0.580 oz) (thus including younger voles). More surprisingly, the long-eared owls were taking voles averaging some 9% larger than those taken by tawny owls.[204] Wild mice, bank voles and, to a more pronounced extent, Microtus voles undergo population cycles over a three-year (or sometimes four-year) span, which frequently requires the owls to alternate their foods when populations decline.[205][206] This effect was studied in the British Kielder Forest and the nearby Kershope Burn. Here tawny owls are exceptionally dependent on field voles (Microtus agretis) as food, constituting about 64.3% of 1220 prey items in the area, but the Kielder forest field vole population had an exceptional four-year drought whereas in the same time frame Kershope kept a more stable owl population seemingly because it retained the typical three-year cycle.[207][208] In Wytham, Britain, tawnys were thought to remove up to one-third of the local population of bank voles and one-third to three-quarters of the less numerous wood mouse (Apodemus sylvatica).[198] On a 148 ha (370 acres) plot of Wielkopolska, tawny owls are thought to remove an estimated 2,213 rodents annually, or 15 rodents per ha each year, which was about the same rate of loss of striped field mouse (Apodemus agrarius) per ha in the Warsaw area as well.[209][210] In Białowieża Forest, tawny owls were estimated to remove in autumn 54% of the yellow-necked mice and 40% of bank voles.[211]
In the largest known European diet studies, rodents usually are predominant. Amongst 68,070 prey items in Slovakia, the main prey were the yellow-necked mouse (23.8%), the bank vole (9.9%) and the common vole (9.14%).[188] In the Czech Republic, the same three main prey species led the foods amongst 17,433 prey items, with the yellow-necked at 33.4%, the common vole at 15.7% and the bank vole at 11.2%.[188] Among prey groups in Grunewald, Germany, with 13,359 vertebrate prey items studied, Apodemus species made up 25.7% of the foods and Microtus voles of about four species made up a further 16.7%.[212] The diet differed in the German area of Herrnut, where the common vole was dominant in the foods at 53.3% of 8513 prey items.[188] In a little over half of about 15 smaller prey studies for tawny owls in Poland, mammals led the food composition of owls by number, but in different areas and habitats of the nation, yellow-necked mice, common voles and bank voles could be at the top of the list.[213][209][214] Of 43,000 mammal prey items in an older large study of central Europe, 66% were bank or Microtus voles, while a further 24% were Apodemus species.[215] In Bourgogne-Franche-Comté, France, amongst 51,743 prey items, Apodemus species, presumably dominated by the wood mouse, made up 51.1% by number and 48.8% of the biomass followed by bank vole, at 20.4% by number and 15.6% by biomass.[216] In western Switzerland, the diet was similar but far more homogeneous, with Apodemus species at 74.3% and bank vole at 18.7% among 10,176 prey items.[217]
The northernmost food study for tawny owls thus far conducted showed that in Sweden, field voles were the main food amongst 578 prey items, at 30.5%, with bank voles being supplemental at 8.7%. However, the second most commonly taken prey in Sweden is the much larger European water vole (Arvicola amphibius), which weighs an estimated mean of 177 g (6.2 oz), and presumably a very nutritious prey resource to these owls.[218] The easternmost food study thus far known was a small one of 201 prey items for the tawny owls in Moscow, wherein the common vole was dominant at 72.6%.[219] Of similar longitude, in the Caucasus, amongst 1236 prey items, the main foods were Ural field mouse (Apodemus uralensis) at 48.1% of the prey composition and edible dormouse (Glis glis) at 15%.[188] The tawny owl takes many species of dormouse, which are nocturnal, largely arboreal and generally rarer within the forests and edges than common mice and vole prey.[220][221] While many dormice are smallish (roughly vole or mouse sized), the edible species is often more than five times larger, being close in dimensions to the European water vole. Therefore, the prey biomass must have been hearty in Montenegro, where the edible dormouse was the main food, at 24.1% of 529 prey items.[188][222] Other more easterly parts of Europe show relatively high balances of edible dormice as well, such as in Bulgaria, Romania and Slovenia.[188][223] Another widely taken species is the hazel dormouse (Muscardinus avellanarius), as well as at least three further species.[188][221][224][225] Another rodent of special interest due to its natural scarcity and its place in the diet of tawny owls is the northern birch mouse (Sicista betulina), which was found to constitute as much as 7% of the foods in some districts of Lithuania, but only contributed 0.6% of the foods overall in the country.[226][227]
All told, no less than 80 rodent species are known to be taken by tawny owls. While most of these are characteristic prey such as various voles and lemmings and any type of murid rodent from the smallest available mice to the largest available rats, other rodents also taken.[7][188] Black rats (Rattus rattus) were noted to be the main prey for tawny owls in Sicily, where they accounted for 35.3% by number of 351 prey items and 60.2% of the biomass, resulting in a relative high mean prey mass of 79.7 g (2.81 oz) here.[228] Strong biomass contributions were noted of brown rats (Rattus norvegicus) elsewhere such as in Lublin in Poland (wherein they accounted for 41.5% of the biomass) and in Algeria (wherein they accounted for about 20% of the biomass), although many rats taken are on the young side rather than large adults, especially of the large brown species.[189][229][230] The tawny owl's prey spectrum also extends to less accessible prey like squirrels (including ground squirrels), with more or less all the species of Europe and western Asia known to be taken by these owls despite their diurnality, as well as the nocturnal but scarce flying squirrels.[111][188][209][231][232][233] The widespread red squirrel (Sciurus vulgaris), estimated to weigh an average of 150 to 300 g (5.3 to 10.6 oz) when taken, appear to recognize the tawny owl as a serious threat, with ones exposed to recordings of their calls recorded to interrupt feedings, engage in rapid movements and scold harshly.[162][189][207][234] Hamsters may too be taken despite favoring and occurring in more open habitats than those usually hunted by tawny owls.[235][236][237][238] In the southerly parts of the range, as they've acclimated to semi-desert, tawny owls can sometimes partially off of quite different murid rodents like jirds and gerbils as well as the non-murid blind mole rats.[188][238][239][240] Rodent prey may range up to the size of probable juveniles of the non-native nutria (Myocastor coypus).[241]

Shrews are a common component of the foods of tawny owls, less so their larger but generally less numerous distant cousins such as moles and hedgehogs. More than 20 species of shrew are known in the foods of this owl.[7][188] While usually secondary, shrews are widely present in the pellets and prey remains in most studies. Unlike some owls such as long-eared owls they do not seem to disdain these musky-tasting and slight insectivores.[6][80] Certainly the most reported variety would be the widespread common shrew (Sorex araneus).[242] Exceptionally, in a large food study for Belgium, common shrews were the leading prey species, at 18.2% of 15,450 prey items.[188] In a much smaller study in Norway during the summer, the common shrew was the leading prey species, constituting 30.4% of 69 prey items.[162] However, given their small size, with the common shrew being one of the larger available species at merely 8 to 11 g (0.28 to 0.39 oz), shrews are a marginal contributor to the owl's prey biomass and taken for subsistence until a more substantial food source is available.[80][189][243] Exceptional quantities of shrews may be predicted in French studies (usually during preferred prey shortages), with shrew prey contributing up to 15% of the biomass overall and more locally, in the Oignies, to 29% of the biomass.[244][245] Despite the low numbers of moles that are usually hunted, species such as the European mole (Talpa europaea) can be contribute heartily to the prey biomass, such as in Wytham, where the species made up 15.6% of the biomass.[7] Although such prey is known to be relatively limited in the species' foods, tawny owls are known to hunt the smallest living mammal species (by weight), the 1.8 g (0.063 oz) Etruscan shrew (Suncus etruscus), up to the size of the largest mole, the 440 g (16 oz) Russian desman (Desmana moschata), as well as perhaps larger still, some small adults of the European hedgehog (Erinaceus europaeus).[189][246][247]
On occasion, tawny owls will prey on young European rabbits (Oryctolagus cuniculus) as well as very young hares. Mostly neonatal or scarcely older rabbits are taken, with a few studies estimating the mean weight as caught as only 100 to 350 g (3.5 to 12.3 oz).[8][189][243] Access to European rabbit was said to cause the mean prey mass of tawny owls in parts of the Netherlands to an unprecidently high 130 g (4.6 oz).[193] One Spanish study claimed that up to 23% of the vertebrate prey for the tawny owl was made up of by rabbits, making them the smallest known avian predator to show a dependence on them.[248] Though generally a minor part of the diet, a wide diversity of bats are taken by tawny owls, with over 30 species in their prey spectrum.[188][249][250][251][252] Usually less than 1% of vertebrate prey consists of bats but in Poland, dietary relations have been studied between tawny owls living near bat caves and urban bat roosts, and locally up to as much as 2% of the diet (and 5.3% of the mammalian foods) can consist of bats. Studies have indicated that bat species are more or less hunted in proportion to their occurrence in mixed colonies and are taken more so within urbanized environments as well as when staple rodent prey is low.[249][251][253] In Austria, 252 attacks by tawny owls were recorded at a colony of Geoffroy's bats (Myotis emarginatus), 31 of which were successful.[254] In Great Britain, it is estimated that tawny owls eliminate at minimum 140,000 individual bats annually.[255] While most bats encountered (and hunted) are fairly small-bodied, tawny owls may hunt bats of all sizes available, from the roughly 4 g (0.14 oz) common pipistrelle (Pipistrellus pipistrellus) to the 59 g (2.1 oz) greater noctule (Nyctalus lasiopterus) in Europe and to the 140 g (4.9 oz) Egyptian fruit bat (Rousettus aegyptiacus) outside of Europe.[239][251][256] Other mammalian prey recorded have been mustelids. The tawny owl is known to hunt both common weasels in Europe, including fairly large stoats (Mustela erminea), weighing averages of up to 200 to 300 g (7.1 to 10.6 oz), despite the potential risk of counterattacking by these bold and powerful hunters.[7][162][243][257] Traces of an even bigger mustelid have been found, the European pine marten (Martes martes), in the foods of tawny owls, though it is a considerable possibility that this was scavenged rather than killed by the owl, much like the verified case of tawny owls scavenging remains of European polecats (Mustela putorius).[7][188]
Birds
Tawny owls do not take birds as commonly as mammals. Unlike the unrelated lineages of diurnal birds of prey, owls in general seldom prefer avian prey, with most varieties preferring small mammals and/or insects, except on a local basis (the closest to a specialized hunter of other birds are some in the pygmy owl genus).[6][4][10] Tawny owls do opportunistically hunt birds through most of the range, however.[6] When it comes to avian prey, there is little evidence that any particular kind is sought out and the owls are likely to randomly come across other birds as an alternate food choice.[7] Usually, European studies show that birds normally constitute less than 15% of the total foods of this owl.[80][188][215] In central Europe within an older study, 6000 bird prey (or a little less than 10% of the recorded prey) items were recorded of nearly 100 species, 33% of which were sparrows.[215] When capturing avian prey, the tawny owls not only pluck the prey but also often decapitate and inflict fairly extensive skeletal damage, especially when the victim is a relatively large bird.[6][258] When a population of great (Parus major) and Eurasian blue tits (Cyanistes caeruleus) was artificially increased by researchers in the vicinity of tawny owl nests, it was found that, despite the tits not being common food, the owls did reduce the population of increased tit. Many more tits were taken during snow cover or while incubating, with male tits being often being taken in larger numbers, and tit numbers were further reduced when fewer rodents were available.[259] In Gothenburg, Sweden, on 26 tawny territories the diversity of songbird species was higher (at 12.83 average species) inside tawny territories than outside (10.3 species). Attempts to study whether songbirds were significant in the foods found that bird altogether amounted to 6.78% of the total prey numbers (most likely to be thrushes), so were not significantly effected here.[260] As is the case with other medium-sized owls in Europe, there is some evidence that local snow cover, arid habitats and/or urbanization will increase the importance of avian prey.[6][80] There was a fairly strong indication of local urban habitat causing the tawny owl to take a large quantity of bird prey in Grunewald. Here, among 13,359 prey items, bird constituted 35.9% with primary prey of this group being house sparrow (Passer domesticus) and European greenfinch (Chloris chloris) and that avian prey was more reliable and productive in the area than rodent prey due to the cyclic populations of the latter.[212] Elsewhere in Germany, in the Pankow borough of Berlin, the house sparrow was also the most regular prey species, at 19.2% of 1912 prey items.[261] The diet of tawny owls in the Polish city of Toruń was also dominated by birds, with them making up 47.8% by number and 51.8% of the biomass against 39.8% of the number and 36.1% of the biomass by mammals. Here the diet was led by the house sparrow (25.9% by number, 22.6% biomass) and secondarily the Eurasian tree sparrow (Passer montanus).[262] In Warsaw, birds were dominant in the city, at 73% of the food. However, much like Toruń, in the rural or outer suburban vicinity (i.e. the Kampinos Forest) outside the urbanized areas of Warsaw, other prey such as rodents and frogs were favored instead.[214][263] The urbanization effect was particularly strongly noted in England, where birds constituted only about 4% of the foods in the countryside per two studies, but in Wythenshawe part of Manchester and Holland Park in London, birds made up 89% and 93% of the foods, respectively. The most important avian foods to English tawny owls were the house sparrow (at 27% and 52% in Wythenshawe and Holland Park), the common starling (Sturnus vulgaris) (at 33% in Wythenshawe) and the rock dove (at 22% in Holland Park).[264] Some urban pairs in Italy derived about 50% of their food from rock doves and common swifts (Apus apus) of all ages, grabbing prey of the two species directly off their building ledge nests.[12] In the Sahel of Algeria, where small native rodents are scarce, birds account for about 39% of the diet tawny owls amongst 2472 prey items, in particular the house and Spanish sparrows (Passer hispaniolensis), at 16.6% by number and 17% by biomass.[229] Birds strongly dominated the foods of tawny owls in the Levant area, such as northern Israel, accounting for more than 70% of 229 prey items, especially Passer species.[265] In the northern stretches of the range, when birds are taken, slightly larger avian prey such as thrushes, often averaging about 80 g (2.8 oz), tend to be taken instead of sparrows and the like.[218][266] In Amsterdamse-Waterleidingduinen area of the Netherlands, birds tended to dominate the biomass especially medium-sized passerines such as common starlings and Eurasian jays (Garrulus glandarius), with these contributing 54% of the biomass in high Apodemus mouse years to 62.7% in low mouse years in March–May. Smaller birds such as birds decrease from 21.1 to 3.1% in the spring while large birds such as pigeons and Eurasian woodcocks (Scolopax rusticola) may increase from 16.2 to 37.7% during high and low years for mice.[193] In a small study from Norway, a large portion of diet consisted of birds in summer (61% of the biomass but only 23.2% by number) while, in winter, voles almost completely dominated the foods.[162]

A huge diversity of birds may be taken by tawny owls, although most are not numerically significant.[264] Slightly over half of the avian prey spectrum for tawny owls are various passerines down to the size of Europe's smallest bird, the 5.2 g (0.18 oz) goldcrest (Regulus regulus).[34][264] At the other end of the size scale for passerine prey are corvids, including jays, magpies and assorted crows.[6][7][264] In some cases, tawny owls have apparently preyed on adult crows of around their own size or slightly larger, such as an estimated 572 g (1.261 lb) carrion crow (Corvus corone).[243] Frequently, the largest prey item found in dietary studies for tawny owls are relatively outsized birds, such as the aforementioned crow, or an estimated 220 g (7.8 oz) western jackdaw (Corvus monedula) in central Italy and a common kestrel (Falco tinniculus) of the same estimated weight in Suffolk.[19][267][268] Although many species of dove are also taken,[188] rock doves and common wood pigeon, the latter taken frequently as adults and estimated to average at 480 g (1.06 lb) when taken in England by two different studies, can be very hearty prey.[145][243] Other large avian prey reported taken as adults by tawny owls (many of which approach or exceed the owls themselves in body mass) includes green-winged teal (Anas crecca),[243] red grouse (Lagopus lagopus scotica),[269] hazel grouse (Tetrastes bonasia),[188] grey partridge (Perdix perdix),[270] chukar (Alectoris chukar)[188] common moorhen (Gallinula chloropus),[188] Eurasian coot (Fulica atra),[4][188] black-headed gull,[271] black-legged kittiwake (Rissa tridactyla)[272] and black woodpecker (Dryocopus martius).[188] Larger gamebirds have been taken such as black grouse (Tetrao tetrix) and common pheasant (Phasianus colchicus) as well as are some large birds of prey are sometimes found in the foods of tawny owls but it is not clear that these adults and may refer only to juvenile individuals.[188][273] Reportage of tawny owls predation on much larger western capercaillie (Tetrao urogallus) is quite likely to refer to juvenile capercaillie.[188] In at least one case, a tawny owl preyed upon an adult mallard (Anas platyrhynchos), which, at a mean weight of around 1,060 g (2.34 lb), is about twice a tawny owl's size and possibly the largest prey known to be tackled by the species.[7][34][176]
Reptiles, amphibians and fish
.jpg.webp)
Little evidence has been found of tawny owl predation on reptiles. Despite their scarcity about a dozen species are known to be hunted by this predator, including a couple species of snake and several lizard species.[7][188][144][240][274] They are more or less taken incidentally, constituting always less than 2% of the foods in known European studies.[9][188][275] An exceptional case was in Sahel, Algeria, where the Moorish gecko (Tarentola mauritanica) was the leading prey species at 16.75% of 2472 prey items.[229] Amphibians are generally much more prominent in the tawny owl's diet, almost exclusively frogs.[7] Nearly 20 species of amphibian are known to be taken, which includes two species of newt outside of the more typical frogs and toads.[7][188][229][276][124] Key to predation on frogs is the composition of the habitat, with frogs and toads being apparently much more accessible in remote and conserved areas rather than developed lands.[7] In different areas of Poland, the Rana genus of frogs led the prey composition such as in Białowieża Forest, where they made up 21.1% of the foods, in Wigry National Park where they constituted 17.4% of the diet and in the northeasterly section of the country where they made up 23.4% of 2046 vertebrate prey items.[270][277][278] Elsewhere frogs and amphibians are regular but secondary foods. Of 3194 prey items in Finland, amphibians (probably all frogs) were secondary to rodents, and could account for from 16.6% in good vole years to 19.5%, the leading prey type by number, in bad vole years, with an average 17.5%. from all years.[279] In Lithuania, frogs constituted 14.5% of 1125 prey items, with the common frog (Rana temporaria) in particular accounting for 11.2%.[280] In Sahel, Algeria, the Mediterranean painted frog (Discoglossus pictus) was a fairly important prey resource, at 9.22% by number and 10.5% by biomass.[229] The average size of frogs taken can be fairly variable, from an estimated 7 to 39.8 g (0.25 to 1.40 oz), as claimed for central Italy and England, respectively.[189][19] There are now several instances known of tawny owls preying on fish, though they are not known to be a significant food source anywhere in the distribution.[7][188] About eight species of wild fish are known to have been captured, including probably young or infirm specimens of large fish such as northern pike (Esox lucius) and brown trout (Salmo trutta), with at least some instances of tawny owls also catching ornamental goldfish (Carassius auratus) as well.[7][188][243][149]
Invertebrates
The tawny owl feeds more extensively on invertebrates than many of the more northerly European studies would indicate.[7] Virtually, any variety of edible invertebrate would be eaten by these owls, though generally insects are taken due to the high occurrence of encounters. Nearly 70 invertebrate prey species have been noted.[6][7][188] In the more southerly parts of Europe, much stronger numbers of invertebrates tend to be detected.[7] In central Italy, invertebrates constituted 53.3% of 654 prey items, in particular scarab beetles (15.8%), land snails (12.1%), ground beetles (5.3%), Orthoptera (5.14%) and longhorn beetles (5%).[267] In the vicinity of Umbria in Italy, 47.8% of 874 prey items were invertebrates, particularly keelback slugs and roundback slugs, which together made up 28.6% of the prey items followed among invertebrates by Melolonthinae subfamily of scarab beetles at about 7% of the prey.[281] In Muránska planina National Park in Slovakia, keelback slugs were the most identified prey type among 13,912 prey items, accounting for 26.1%.[282] Keelback slugs were also the main prey type in Bulgaria (accounting for 24.6% of 529 prey items), and detected in strong numbers in Romania, the Caucasus and Crimea (wherein they made up 21.8% of 514 prey items, but trailed by number the yellow-necked mouse).[188] A strong dominance of insect prey was detected in food studies from Spain, with 64.3% of 1002 prey items from across the nation being invertebrates. In the Spanish region, the wood mouse was the identified single prey species (at 20% by number and 21% by biomass) but was closely followed by bush crickets (at 19.76% and 1.5% biomass), Rhizotrogus aestivus (10.76% by number, 0.5% biomass), European field cricket (Gryllus campestris) (8.85% by number, 1% biomass), minotaur beetle (Typhaeus typhoeus) (7.92% #, 1% biomass) and common dor beetle (Anoplotrupes stercorosus) (4.35% by number, 1% biomass).[8] More locally within the Province of León, beetles collectively constituted 35.1% of the diet and Orthoptera constituted 14.4%.[274] In Sahel, Algeria, invertebrates in total slightly outnumbered mammals, but lagged slightly behind birds in number.[229] In general, insects in central and northern Europe are a regular but secondary food source, taken in similar volume to birds but far less significant as contributors to biomass.[6][7][169] Tawny owls are said to take beetles in central Europe more frequently in April–May before ground cover becomes too extensive.[283] An exceptional case of invertebrates being primary as a food source in a northerly country was recorded in the Peak District of England, wherein earthworms were the primary food type, at 40.4% of 926 prey items and 15.6% of the biomass. Strong numbers were detected here too of Geotrupes beetles, contributing 14% of the prey numbers.[269]
Interspecies predatory relationships

In every part of its range, tawny owls co-exist with other birds of prey, with other owls presenting the strongest possibility for competition given their overlapping food selection and shared nocturnality. As perhaps the most numerous and one of the most widely distributed in the continent of the 13 owl species regularly occurring in Europe, ecological interactions of some kind have been recorded with most other species.[6][80] Given their medium-sized frame and general adaptability, of special interest is how they co-exist with other medium-sized owls such as long-eared owls and barn owls.[6] Many studies have contrasted particularly the food habits of long-eared owls living in proximity to tawny owls. Generally speaking, the long-eared owls in Europe are much more strongly disposed to being a specialist species than the tawny owl, relying almost entirely on voles. Although in the broad picture, the long-eared also feeds on other prey such as birds and insects, their food niche breadth is consistently lower than that of the tawny owls. For example, in a very large study of central Europe, the common vole species alone constituted about 76% of 57,500 prey items for long-eared owls.[284][204][285][286] Long-eared owls also differ strongly from tawny owls in selecting much more open hunting grounds, such as old fields, usually hunting on the wing rather than from a perch and in utilizing abandoned (and often rather open) bird nests rather than natural cavities as nesting sites.[6][10][287] In terms of peri-urbanisation, the long-eared and tawny owls are more or less equally adaptive to such areas.[80] The food niche breadth is usually greater in Europe for the tawny owl than for the barn owl as well,[229][238] although the barn owl appears to have a stronger liking of shrews as prey than does the tawny owl (shrews more than twice as often selected).[80] The barn owl, although also by nature a cavity nester, does not generally acclimate to well-wooded areas where the tawny owl is at home.[10] Both the long-eared and barn owls prefer voles where they are available, especially as both often hunt in open areas where they are common, whereas Apodemus mice tend to be slightly preferred by tawny owls.[6][80][288] When conflicts ensue, the tawny owl tends to dominate these other medium-sized owls. This is in part due to their size advantage, with the tawny being larger by an average of 32% (of 3 standards of measurements, two from the wing, one by body mass) than the long-eared owl and 24% larger than the barn owl.[6][289] Tawny owls are known to readily evict barn owls from their own nest sites, normally when taking up residency in towns or cities.[133] It was additionally found that barn owls, being a species better adapted to warmer, more tropical areas, is at higher risk of starvation in cool weather than long-eared and tawny owls, with proportionately many more found dead in winter in France due presumably to inferior lipid fat reserves.[290]
In the British Isles, the tawny owl is the largest and most powerful year-around native owl, ahead slightly of the long-eared and barn owls. Therefore, the tawny owl may be considered an apex predator here (despite still being vulnerable occasionally to diurnal raptors and ground predators). However, since the tawny owl never colonized Ireland, here the long-eared owl is the largest year-around owl (in these island much larger owls are very rare winter visitors, in the case of the snowy owl (Bubo scandiacus) or are probably accidentally introduced by humans, as is likely the Eurasian eagle-owl).[6][78][269] However, in much of mainland Europe and elsewhere, tawny owls potentially overlap with larger owls and, depending on habitat composition and prey accessibilities, may be considered more correctly a mesopredator.[6][80] Mainly in parts of northern and eastern Europe, tawny owls sometimes co-exist with their larger cousins, Ural owls. The Ural owl has generally similar nesting and feeding habits but tends to occur in slightly different habitats. Generally, the Ural is more broadly adaptive to taiga and similarly conifer based forests than are tawny owls and is also somewhat more likely to be active during daylight.[80][136][218] In eastern Europe, the Ural species tends to occur more so at higher elevation in montane forest such as the Carpathian Mountains, especially those with extensive old growth of beech trees, while the tawny owls tend to occur at lower elevations and more mixed forest with fewer glades in these areas.[291][292][293] A relatively low territory overlap of 13.3% was detected in Slovakia between tawny and Ural owl territories due to their differing habitats.[294] Depending on range, the prey sizes taken by tawny owls tend to be considerably smaller than those selected by the more powerful Ural owl, with the latter's mean prey sizes averaging from 31 to 50% larger. However, the food niche breadth is up to two and a half times greater in the tawny than in the Ural owl.[136][266] The Ural owl tends to dominate interspecific conflicts with tawny owls.[136][266][292] On the contrary, in at least one case a tawny owl was observed to fiercely attack and drive off a Ural owl (although it may not be ruled out that this was a case of mobbing).[6][291][292] A third and much larger still Strix species, the great grey owl, differs considerably in almost all respects of its life history from tawny owls. The great grey is adapted to taiga and other conifer based forests, both open and enclosed, and relies almost exclusively on voles for food. Almost cathemeral in activity, the great grey species may nest in a broad variety of situations in the boreal habitat but never utilizes tree cavities as does the tawny. Due to the latter species' specialization, the tawny owl is spared from any known ecological interactions with the great grey owl.[136][266]
Much more dangerous than larger Strix species to the tawny owl is the Eurasian eagle-owl. A similar wide-ranging generalist, the eagle owl most often nests in and around rock formations, often in fairly mountainous areas, but locally is also adaptive to varying habitats and may too nesting in old birds nests or on the ground, usually between the trunks of large trees.[6][10][295] In terms of their dietary habits, the eagle owl appears to be perhaps an even more indiscriminate predator, attacking animals of all taxonomic classes unfortunate enough to encounter them. Given its far larger size and much more powerful features, the eagle owl will attack much larger prey than tawny owls, even relative to their own size.[191][295][296] Tawny owls are likely to avoid encounters with eagle-owls and are fortunate in many areas that the eagle-owl, which requires a larger home range and tends to more exclusively prefer remote areas than tawnys, can be scarce to absent in some parts of Europe.[80][295] In some areas of Spain and Italy, tawny owls have adapted to live in the vicinity of wooded montane areas and even to nesting within rock formations. Both countries have healthy recovered populations of eagle-owls, so tawny owls appear to locally restrict their vocal activity and tend to occur on the fringes or outside active eagle-owl ranges.[147][297][298] Unlike their larger, more powerful cousin, the Ural owl, the tawny owl is not infrequently vicim to predation by larger raptors.[299] There are at least 300 recorded instances of predation on tawny owls in Europe by Eurasian eagle-owls.[6][80][299][300] They tend to be taken somewhat less than other medium-sized owls, especially long-eared owls, by eagle owls by virtue of using woodlands (which differ somewhat from the habitats usually used by eagle-owls) and nesting in tree hollows.[80][299] The other greatest predatory threat is certain to be the northern goshawk (Accipiter gentilis).[11][299] There are at least a hundred cases of goshawks taking the owls and, unlike the eagle-owl, the habitats of the goshawks do fairly closely mirror those of tawny owls with the owls only spared by its different primary times of activity.[80][299][301]
Other predators long known to have taken tawny owls have included their larger cousins, the Ural owls as well as common buzzards (Buteo buteo), red kites (Milvus milvus) and peregrine falcons (Falco peregrinus).[80][299] In addition, more reported raptorial predators have included the Bonelli's eagle (Aquila fasciata),[302] golden eagle (Aquila chrysaetos),[303] eastern imperial eagle (Aquila heliaca)[304] and black kite (Milvus migrans)[305] Outside of traditionally raptorial groups, birds such as corvids may destroy and/or compromise tawny owl nests, either for food, anti-predator behaviour and/or competition. Western jackdaws, in particular, appear to be persistent competitors for nest sites and sometimes are aggressive enough as to displace tawny owls from a disputed site. In extreme cases of competition with jackdaws, the owls may bring themselves to starvation trying to incubate their nests in the hole when a murder of jackdaws continuously visit, harass and place a new nest on top of the owl's eggs repeatedly. In other cases, the owls nestlings have been suffocated by the jackdaws building a nest directly on top of the still living owl broods.[6][11][306] Mammalian predators are a fairly frequent threat to tawny owls as well, though tend to attack almost exclusively during the breeding season.[7] European pine martens are known to be a considerable threat of all aged tawny owls at nests from nestlings to brooding females, as are probably stone martens (Martes foina).[307][308][309] In a food study in France, 9% of the diet of pine martens was found to consist of tawny owls, with the data indicating that owls using nest boxes are more vulnerable to martens.[310] Especially once reaching or around the age of fledging, red foxes (Vulpes vulpes) are known to take several young tawny owls (and perhaps an unwary adult), at times taking up to 39% of young owls in a population, as are probably cats (Felis silvestris) in some areas.[80][309] However, in chance encounters during the day, tawny owls have been known to attack and successfully chase off pine martens and have been seen to do the same to red foxes, cats and dogs.[6][311]
The tawny owl is a considerable predator itself of smaller owls. Data indicates that it is second deadliest owl to the smaller species of owl in Europe, behind only the eagle owl.[6][80] Among their known owl prey species are Eurasian scops owls (Otus scops),[188] Eurasian pygmy owls,[312] little owls (Athene noctua),[6] long-eared owls[7] and boreal owls (Aegolius funereus).[299] Additionally, they may hunt smaller diurnal birds of prey such as Eurasian sparrowhawks (Accipiter nisus),[6] common kestrels (Falco tinnunculus),[268] Eurasian hobbys (Falco subbuteo) and merlins (Falco columbarius).[299] Reports of tawny owls killing common buzzards and northern goshawks are of nebulous detail and may refer in fact to nighttime nest robberies rather than overpowering adults of these larger, dangerous and often seemingly avoided raptors.[6] Evidence from Slovenia has indicated that the tawny owl is more feared by small owls such as the boreal owl than even the larger, more powerful Ural owl, as they clustered more strongly as can be explained by habitat in the realm of Ural owl territories but seemed to avoid where possible tawny owl territories.[291][313] Although there are more known instances of tawny owls hunting little owls, data in central Europe could not distinguish whether little owls were avoiding tawny owls or the wooded habitats they frequent to account for their sometimes spotty ranges.[314] However, when forced to nest in quite close proximity to tawny owls and other medium-sized owl species due to clustered "islands" of habitat remaining in southeastern Poland, the productivity of little owls appeared to lower.[315] Predation by tawny owls can be severe as well on Eurasian pygmy owls, to such an extent that they may have cause the regional extinction of the pygmy around World War II in the Black Forest after the smaller species was already depleted by deforestation. A successful reintroduction of Eurasian pygmys into the forest was followed by a natural range expansion back into the forest by tawnys, which again threatens the population growth of smaller owl.[4][316]
Breeding
_(8403003812).jpg.webp)
Tawny owls are monogamous and territorial year around. Young birds select territories and look for mates in autumn and tend to be very vocal, especially males.[6][4] Due to their highly territorial behaviour, young birds frequently struggle to establish a territory unless a nearby adult dies. Males routinely engage in territorial fights.[6][4][10] Territories have been known to have been maintained by single tawnys for up to 10 years in Russia and 13 years in Berlin.[111][46] Of 34 males in Wytham, only one male moved off of territory, due to being disturbed by humans.[45] It appears to be largely up to the male to select territorial boundaries.[6] Despite the aforementioned territorial behaviour, active nests of two separate pairs at as close as 100 m (330 ft), in the Tegel forest, have been reported.[6] This species shows very little extrapair parentage.[317] In Switzerland for example, a study of 137 nestings found that only one, or 0.7%, were from a different father than the mate, females cannot generally raise young without male contribution so the pair structure of these highly residential owls insures little instance of cuckoldry.[318] Cases of bigamy were reported at Wytham in 6 of 34 males, in situations where apparently a neighboring male died and was suffixed subsequently, however, one or the other nesting attempts would completely fail each time.[45][319] In Pavia, 3 of 22 territories included two mature females.[52]
Nests
.JPG.webp)
The male advertises several potential nest sites to his mate by singing at the entrance, slipping inside and so on, with the female finally selecting one.[6] The typical nest site of a tawny owl is a tree hollow, wherein the owls will nest directly on the interior hole's surface. Tree hollows used may be as much as 25 m (82 ft) above the ground, but are usually within about 12 m (39 ft) of the ground.[7] Virtually any species of deciduous tree may be used provided holes are available. These tree cavities may be of any origin, with trees that grow large such as oak, beech, poplar, maple, lime, hornbeam and alder often regularly utilized.[149][320][321][322] Female may scratch out a shallow base in soil if present and sometimes seen to reportedly tear eggs into pieces as a cushion from their broods.[10] Othe tree nest locations have included those on top of a Witch's broom and on top of the tree canopy.[323] Natural holes in trees are often the most frequently used nesting site, followed closely in recent decades many artificial nest boxes, preferably those with a 15 cm × 20 cm (5.9 in × 7.9 in) entrance or larger.[6][324] Of the nest boxes erected in different parts from Kielder forest and the Glenbranter forest of Argyll, 592 nest boxes were placed at 1.6 to 5.2 m (5.2 to 17.1 ft) high along the side of trees and 17.4% of which were used by tawnys (in latter 2 years of study up to 24.1%).[325] In southeastern Scotland, all nest boxes erected in habitat were eventually utilized by tawny owls.[326] Many nest boxes were recorded to be used as roost sites in the Milan area, with only 12.3% of the 44% of nest boxes actually used by owls for breeding, usually with the owls utilizing boxes that were at least 6 m (20 ft) above the ground.[327] Nest boxes are most successful wherever natural tree cavities are scarce or absent, such as conifer forests, young successional woods and farmlands.[207][328][329] Tawny owls may not infrequently nest in an unmodified black woodpecker hole.[137] This species may too nest in nest of larger birds such as crows, common ravens (Corvus corax) and Eurasian magpies as well as common buzzards, black kites, northern goshawks and various eagles while the sometimes recorded as used smaller nests such as those of Eurasian jays, Eurasian sparrowhawks and common wood pigeons but these are at potential risk of collapse.[6][4] Occasionally, tawny owls have also been recorded nesting in abandoned burrows of larger mammals (e.g. red fox and European badger (Meles meles) as well as those of rabbits).[6][4] Other nesting locations recorded for the species have included bare cliff ledges, between the roots of heavy tree trunks, on the bare forest floor and among heather. Also rarer still nests have been reported in the recesses of stone walls, in chimneys of large buildings, on cabins and sheds, in dovecotes and church towers.[6][4] In southern Finland, 95% of 123 nest boxes put out were occupied by tawny owls in 1970–1975, natural tree hole use decreased in same period from 48% to 3%, on top of stumps from 4% to 1% and in buildings from 15% to 1%.[80]
Eggs

Laying normally begins in March-early April, sometimes as early as February.[4] Of 344 nests in Latvia, the mean egg laying dates were March 13 to April 3, with extreme dates of February 20 to April 30, with shifts accounted for by late winter weather.[330] In the German area of Swabian Jura clutches may start to be laid as early as mid-February if food is favorable.[331] Egg laying in mid-February is fairly typical in Italy as well.[7] The first egg is typically laid by mid-March in Great Britain, in late March in continental Europe and early April in Scandinavia.[6] In very snowy years in Finland, the laying of clutch is often either delayed or does not occur at all.[332] Due to the warmer average ambient temperatures and access to pestilent rodents, wintertime egg laying in human developed areas is now known to be commonplace: with records of laying in late December in Amsterdam, early January in Munich and late January in Riga, Latvia.[6][331][333] They tend to lay their clutches earlier than long-eared owls and little owls and much earlier than barn owls.[6][10] One clutch is laid per year but rarely replacement clutches have been reported if an entire clutch is lost.[7] A record in Bizkaia, Spain of a second successful clutch was produced, with pairs of owlets were recorded in February then another pair of owlets apparently hatched to the same pair was recorded in July of same year.[334] The female typically lays a relatively small clutch of 3-5 eggs, sometimes in extreme case from 2 and very rarely and in the times of food plenty, 7–9.[4][10] Average clutch sizes were cited as 2.67 in Britain, 2.8 in Bohemia, 2.95 in Belgium 3.29 in central Europe, 3.3 in both Switzerland and the Netherlands, 3.65 in Denmark, 3.64-3.81 in Finland and 4.04 in Sweden.[6][80][45][158][329][335][336][337] While in Bohemia, the clutch size varied remarkably little by year, in western Switzerland it could go from 2.52 to 3.63 in different years and Finnish data indicates it can vary from 3.1 to 4.2 in low and peak vole years. Therefore, clutch size may be more variable in more cool climatic zones but, on the other hand, the average size of the clutches averages larger in these colder areas.[80][329][336][338][339] Although clutch sizes average smaller in Great Britain than in mainland Europe, they are more consistently laid there than the clutches of barn owls.[10] Their eggs are pure white, smooth or slightly glossy in texture, and vary little in size. Egg dimensions were found to average 46.7 mm × 39.1 mm (1.84 in × 1.54 in) in Britain, 47.6 mm × 39.2 mm (1.87 in × 1.54 in) in central Europe, 46.6 mm × 38.5 mm (1.83 in × 1.52 in) in Sweden (Makatsch 1976) and 47.5 mm × 39.2 mm (1.87 in × 1.54 in) in Russia. The range may be from 42 to 50 mm (1.7 to 2.0 in) in height by 36 to 41 mm (1.4 to 1.6 in) in diameter (sample size 100).[6][7][111][340] The average weight of eggs can vary from 31.3 to 40 g (1.10 to 1.41 oz).[6][4]
Parental behaviour
The female incubates alone, starting with the first egg for 28–29 days typically, and is fed by the male.[4] The female also broods the chicks, feeding them strips of meat for about 2 weeks, after which they may start swallowing mice and such whole. She continues to feed them until they leave the nest, at which point she may resume her hunting but the male may continue to a majority of food capture and may even feed the offspring directly.[6][4] Larger prey items tend to be more often fed to chicks such as medium-sized birds, young rabbits and moles where available, while the parents themselves usually eat small rodents (i.e. in Wytham and Ythan Valley).[164][341] Sometimes when the mother feeds their young, separated feathers are sometimes doled out and consumed by them.[135] In France, 4–19 prey deliveries by the male were recorded per night, or 2.5–3 prey per chick nightly.[216] In Belgium, from as little as 0 to 22 prey items were recorded to be delivered per night, on average 3.7 (range 2.3–5.1) prey per chick nightly.[342] Up to 21% of prey deliveries were done in daylight in Wytham whereas in the Netherlands only 2% were during daytime.[18][343] In one British nest, with three owlets (the oldest being 15–25 days old) each chick received about 88 g (3.1 oz) of food per night while later near fledging, each would get 124 to 141 g (4.4 to 5.0 oz) per night.[344] In an experiment study, broods were both increased and decreased to see if males differed their food deliveries, it was found that the males did change their food delivers as a result of brood increase or decrease, with the decreased broods being fed well while the increased broods were food stressed and showed particular signs of declining condition of the brooding female as she had less food for herself.[345] Instrinic factors were weighed about the condition of parents in a study from Finland, with the female's age being the most significant factors that could be determined. Furthermore, it was found that male condition was the most important factor for female condition, with older males often in better physical condition.[346] Weather conditions were found to be intrinsic with condition of parents in Finland, and further that larger females could breed earlier and more successfully on average.[347] A study of steroid hormones in a selected 51 pairs within Denmark (from a network of 204 nest boxes) were used to measure hormone levels. It was found that testostrone levels were consistently higher in 3 year old birds of both sexes, birds of this age were more productive in all aspects of breeding than younger owls, although it was not clear to what extent higher breeding success to attributable to hormonal levels versus experience.[348] A testing of testosterone levels in owl offspring within Danube-Ipoly National Park in Hungary determined that experienced parents produced nestlings with more testostrone mainly in correlation with mild weather. In poor weather or inexperienced parents the levels were lower. The testostrone levels in the Hungarian study were found to be lowest in poor weather conditions in the last of the owl's broods and most such young usually died during the early nesting period.[349]
%252C_Parc_de_Woluw%C3%A9%252C_Bruxelles_(23814972790).jpg.webp)
When they are alarmed or required to act in self-defense near the nest, tawny owls of all ages but especially older nestlings up to fledging age, have been recorded to raise feathers and outstretch wings, to their expand size and possible shield itself, also snapping their bills.[350] Despite the tawny owl's reputation as a highly aggressive bird when encroached upon,[46] the parents are usually retiring even when the area of the nest is broached.[6] Passive measures are usually taken first in parental nest defense.[7] The mother owls are often tight sitters, one female sat motionless even as she was physically turned over, while another sat tight but flushed eventually after several human intrusions.[351][352] Sometimes parents engage in distraction display when attempting to protect their young but less frequently than do long-eared owls.[331] There is much individual variation in aggressiveness of response to disturbance and threats, with a similar occasional but widely reported tendencies for aggressiveness in nest defense in many other Strix owls as well.[6][7] In the earlier part of the nestling period, males sometimes launch defensive attacks but these tend to be relatively mild and no physical contact is often made.[6] In western Switzerland, females were 2.9 times more likely to respond aggressively to artificial nest predator stimuli than males.[353] Later into the nesting period, the female may begin showing more aggressive reactions to disturbance or threats.[6] Older females were found in a study show a more aggressive defense as well as those with larger broods and earlier nestings, however non-aggressive females were found to have more future reproductive years on average than aggressive ones.[7][354] Rufous morph females were more vigorous in nest defense than other morphs in a study from Switzerland.[353] At the first detection of an intruder, the female may let out muffled hoots initially.[157] Attacks only tend to occur somewhat regularly in developed areas, especially city parks, where repeated intrusions occur and perhaps resulting desensitization and irritation.[44][46] Some individual females and rarely males become routine attackers on humans and may be known as "furies".[158][354][355] In at least some cases parks may be closed due to unprovoked tawny owl attacks.[356] The female tends to attack humans from behind and focus on the head and shoulders when physical contact is made, especially if the person turns their back, but even then often veer short of physical contact. Humans are inefficient at fending off sudden physical attacks because of the silent flight of the owls.[43][157][357][358][359] Tawny owl mothers not infrequently attack threatening animals common in parks such as dogs and cats as well as martens (at times knocking them out of the trees) and red foxes, especially at dawn or early in the night.[6][43][311] Other than scalp abrasions, occular damage can be considerable when tawny owls attack humans.[43][357] Perhaps the best-known victim of the tawny owl's fierce attack was the renowned bird photographer Eric Hosking, who lost his left eye when struck by a bird he was attempting to photograph near its nest in 1937. He later called his autobiography An Eye for a Bird.[360]
Development of young

Young begin to call in about 24 hours before they hatch.[4][7] Asynchronous hatching occurs but is slightly less pronounced perhaps than long-eared and barn owls, rarely ranging up to 2–3 days apart.[6] The female broods the owlets closely until 10–15 days, rarely ceasing as early as 7 days.[4] In a Danish study, it was found that 59% of 268 nestlings were male, as opposed to roughly even sex ratio in Great Britain or Hungary, with the ratio not changing annually unlike clutch size, brood size and reproductive success.[361] The gender of broods were studied relatively to testosterone levels in differently sized clutches in Hungary with smaller clutches with lower testosterone levels being male-biased. In this Hungarian study, survival rates were higher in smaller broods than in larger brood. Heavier parents raised all offspring hatched to them, while lighter parents raised 33% of the offspring. 59 of 99 reduced broods were males, while 34 of 81 unmodified broods male.[362] Although sometimes said to only fed young at night,[344] the mother can also parse out tiny bits of food by day to their nestlings.[80] The young are fed small bits of meat for about 12 days, at which point the young open their eyes and begin to more actively beg.[7][190] Also around 12 days, the nestlings produce their first pellets, though they are often of a rather liquid consistency.[157][244] Older nestlings beg with quivering wings and intense high-pitched food begging calls.[43] Female only regularly hunts again after brooding period (usually a little after the young are 2 weeks old).[7] The male tends not to enter the nest to make food delivery, often the female receives it nearby on a branch of the tree or at the entrance of the nest hole, later when the nestlings are large, the male often will silently deposit the prey directly into the nest without landing.[43][46] When they are 21–25 days old, the young are stronger on their legs and feet and begin to spend much time around the entrance of the nest hole. Subsequently, they begin to emerge fully about 3–5 days later.[4][46] It was found in warmer conditions, that the owlets born to rufous morph mothers requires less oxygen consumption and may experience lower stress levels during their early development.[363] Evidently the oldest is usually the first to leave hole.[364] After leaving the nest and becoming "branchers", the young owls often clamber around using both the feet and the beak, and often land on the forest floor, from where they tend to flutter and climb into bushes, trying to reach higher parts of the trees (and should not be handled if found on the ground as such).[4][64][157] Finally, at 29–37 days, with an average of 32.1 days in Kielder Forest, the young fledge, but take about another two weeks before they can fly strongly.[64][198][365][366] The young post-fledgling owls continue to beg, often following and rarely leaving an area of about 50 m (160 ft) away from their mother.[367] The pre-dispersal young used an area of 5 to 15 ha (12 to 37 acres) in England while, in Scotland, it was only 2.2 to 6.5 ha (5.4 to 16.1 acres).[365][368] In Denmark, the distance between post-fledging siblings ranged from 11 to 0.6 m (36.1 to 2.0 ft) during day and 32 to 6 m (105 to 20 ft) by night, meaning that they are associative with one another at this stage, and they would spend 20-80% of nighttime hours food begging, up to 82% in poor food years.[369] At around 1 to 3 months (sooner in Denmark, later in England on average), the young tawny owls begin to hunt for themselves.[6] At the age of 2 to 3 months the young owls can be evicted by their parents, although often appear to disperse independently.[4][365][367] However, on average of 72 radio-tagged juveniles in Denmark after the young owls would stop begging for food at 90–123 days of age, they would typically roost within their parents territories for another 18 days without incident.[370] In the Danish Gribskov forest, 41 radio-tracked broods were dependent after fledging for a mean of 71 days (with a range of 56-84).[371] 5 of 12 radiotagged juveniles survived dispersal in a different study from Denmark, independence was gained at an average 77.3 days and single day movements recorded of up to 4 km (2.5 mi).[372] 22 radiotagged young tawny owls in England were tracked post-dispersal relative to vole concentrations, though there was no evidence that there were consistently able to access the peak vole areas. 27.4% of area selection was found to be likely correlated to vole access.[373] The age at first breeding may be early as one year, but is more commonly 2–3 years old and rarely not until 4 years old.[7][157]
Breeding success
_recently_fledged_(1).jpg.webp)
Breeding success averages fairly high in this species. A study within oak-hornbeam-birch forest in Hungary on the breeding output of males, 77 males examined from the 1st breeding year until 9 years of age, increase breeding output from 2 to 4 years of age thence peaking at 5–6 in age. However, at older than this the male breeding output begins to decline, possibly due to senescence, perhaps since males may be unable to continue withhold high-quality territories from competition. The 5 to 9-year-old males were flexible to prey variations in ways younger males were not.[7][374] In Hungary's Pilis Biosphere Reserve during 1992–2000, the age of females appeared to effect the number of eggs and hatching success, while the age of the male effected the number of fledglings, with 39 pairs studied. 98 females and 85 males were produced in a network of 180 nest boxes. Again older males and females (i.e. 5 to 9) were seemingly more productive overall.[375] Hungarian data shows lower overall number of young during years with harsh, snowy winters but due to the low number of young, the successful raised young were stronger and in better condition on average once fully grown than the young owls in years with many offspring.[376] The breeding success overall in Lithuania has steadily increased, with a survival rate reached of 72% for females.[377] The area of Burgundy in France, productivity of successful pairs averaged 3.2, varying from 2 to 3 to 4 in poor, intermediate and good prey years.[378] In western Switzerland, over the course of 14 years, females produced an average of 5.67 fledglings.[379] On an area of 3,800 ha (9,400 acres) in the Netherlands, 9 successful breeding attempts were recorded, a very low density to such an extent that unlike many other parts of the range that attempts to attract owls with playback were largely unsuccessful.[337] As for breeding success near Rome in Italy, out of 326 attempts, 4-28% were successful in different years, with the number fledglings ranged from 0.4-1 overall and 1–1.18 per successful pair and habitats with more rainfall and less arid conditions being more productive.[380] Of 311 breeding attempts studied over a 13-year period in Rome, 59.5% failed in urban plots and 51.3% in suburban areas, with 18.5% and 23.4% in urban and suburban zones producing 1 fledgling, 12% and 18% producing 2, 8% and 7.2% producing 3 and 2% in the urban area producing 4. In Roman areas, the breeding rates are generally quite low but are fairly stable annually, due to warmer average ambient temperature and less local trophic competition. The total breeding success in Roman pairs was 43.5% in the city (also 0.83 fledglings per pair and 1.86 per successful pairs) and 51% in suburbs (also 0.82 fledglings per pair, 1.63 per successful pair).[381] Of 256 Belgian eggs, 24% did not hatch, 10% of those were deserted, 51% were adled and 39% were deserted or destroyed by the female when food supply was low. In the Belgian data, of 195 young hatched, 94% fledged and 6% died in nest as a result of starvation, with an average number of fledglings 2.06, varying from 0 to 3.25 on average in different years based on vole numbers.[157] In southern France, brood size for all pairs was 2.2 while for successful pair it was 3.2 (range 2–4.3 per pair). In the French study, cannibalism was reportedly surprisingly often to be committed by mother or siblings. 73.7% of the studied French broods produced fledglings.[382] Of 357 pairs in the woodlands around west Berlin in 2 decades starting 1958, 160 pairs produced 333 fledglings, with an average of 2.08 per successful nest. 13 pairs in the city parks of west Berlin produced 47 fledglings, 3.3 pairs per successful nest, productivity strongly correlated to number of yellow-necked mice available.[383] More broadly in the Berlin metropolitan, nesting success averaged 2.1 and 2.8 per successful nest, but could vary 2.7 to 3.2 on average in low and high vole years.[331] In eastern Bohemia, an average 2.6 young fledged per successful pair, but no determinable difference in productivity was noted during good and poor prey years.[329] Breeding success varied in Finland based on vole numbers, from 2.4-2.7 in poor years to 3.4-3.5 in good years, but only a slight variation was recorded in clutch size and young hatched.[279][332] Finland has shown a sharp increase overall in tawny owls in the recent three decades was from 422 to 1710 active territories found, from 198 to 1566 nests found and from 168 to 1341 successful nests found. When vole populations were high, the number of young tawny owls introduced into the population of Finland was ten times higher than in low years.[384] It was found in Denmark that a control group of 32 out of 131 radio-tagged young that were supplementally fed by researchers were more vulnerable to predation (36% of these died, mostly due to mammals like foxes around fledging age) but also earlier nests were vulnerable as well (more so to other birds of prey).[309]

Of 562 eggs laid in Great Britain, 314 young hatched, with 44% lost before hatching and 2% lost before fledging.[44] Cambridgeshire produced an average of 0.3 for all territories in fragmented woods and 0.89 for all territories in continuous woods.[7] This study of the English countryside showed that the owl population varied relatively little in proportion to the sharply cyclic nature of the main prey here, field voles and wood mice, due to the owl's ability to exploit alternate prey in poor rodent years.[44] In Scotland, perhaps with less diverse prey available in the more northern clime, the trends of tawny owls were more sharp: 2.6 fledglings were produced in good vole years, 1.65 average in declining years and 0.2 in poor vole years over a 7-year period.[385] Scottish data showed nestling mortality was higher in poor vole years, at about 31% vs 14% in good vole years, with no sex bias.[386] During low prey years in Wytham, anywhere from 33 to 77% of eggs produced are abandoned and may freeze, but little to no change occurs in territory occupancy.[45] Authors inferred that the parent owls in Kielder Forest are able to biological predict the vole numbers based on spring feeding access and produce more often the more productive sex, which were females here.[386] Territorial changes in Kielder were associated to habitat and land use changes with 25% more territories recorded from 1981 to 1991 then a 21% reduction from 1992 to 1998.[387] In this area, due probably to the exotic conifer dominated forest, owls were quite dependent on field vole numbers in the openings, and could vary from twentyfold from low to high years, with 70% of the variance of productivity could be correlated to the habitat types available and show the importance of habitat heterogeneity.[385] In peak years at Kielder, of 197 attempts, 6.9% failed to breed and 28.3% successfully bred, in the increase years, of 44 attempts, 10.2% failed to breed and nesting successful was 76.2% and in the decrease years, of 62 attempts, 12.5% failed to breed and nesting success average was 69%.[387]
Longevity and natural mortality

Tawny owls can live to more than a decade. The oldest recorded in the wild in central Europe was 18 years and 7 months old while the oldest in Sweden recorded was nearly 14 years old.[133] These records were subsequently broken by a female recorded in the wild in Switzerland at an age of 21 years and 11 months.[388] The species has been recorded to live to 27 years or so in captivity.[4][133] If voles are scarce and weather harsh during winter, many tawny owls die by various means (starvation being primary).[10] Starvation rates are high in newly independent young if there is no unoccupied territories in the vicinity (at least by 1 bird of their corresponding gender).[6] Mortality averaged about 15% in territorial adults in Wytham.[45] In Finland, Ural owl displaced tawny owls but great grey owl peaceably allow them in the vicinity.[389] Although moderately hardy during sub-freezing winters, severe winters can be dangerous in areas such as the Russian part of their range.[111][133] Mortality in general in the northern limits of the range is probably higher in the more temperate zone adapted tawny than it is in the Ural owl.[390] This species is increasingly affected by avian malaria, the incidence of which has tripled in the last 70 years, in parallel with increasing global temperatures. An increase of one degree Celsius produces a two- to three-fold increase in the rate of malaria. In 2010, the incidence in British tawny owls was 60%, compared to 2–3% in 1996.[391] Direct anthropogenic causes of mortality are covered later. Of 22 radio-tagged young tawny owls in Kielder, 36.4% (8) owls died 10–106 days after fledgling but while still on parent's ranges, another 22.7% (5) died after leaving parents territory at 40–147 days after fledgling, 22.7% (5) also disappeared after fledged but while still dependent and were quite likely preyed upon while the only 4 remaining lost radio contact after leaving parent's range at 58–178 days after fledging. Of 13 certain to have died, 6 were due to starvation, 3 were due to predation by goshawks, one by an infected eye injury, while the rest of uncertain cause.[366] Mortality rates can be even higher elsewhere such as an average of about 92% in Scotland and about 61% in Norway for juveniles before they disperse.[368][367] Meanwhile, recorded average mortality rates in the 1st year of radio-tagged tawny owl lives were recorded as the following averages: Sweden at 71.7%, Switzerland at 49.4%, England at 52.6%, west Germany at 48% and Belgium at 58%. Radiotagging studies showed average mortality for Swedish 2nd year owls was 44.3%, 24.5% at this point for Swiss owls and 22.2% for English ones while in the third year the Swedish one's mortality was estimated to average 47.6% and English ones at 31.8%.[7] The survival rate average over two decades was unexpectedly somewhat high in Finland in about 20,000 owls, where it was 33% in the 1st year, 64% in 2nd year, 73% in subsequent years.[392] In England, it was estimated that there was 6-11 times lower survival rate for tawny owls that were ragiotagged than for those that were not based on estimates, the theory as to why it lower survival is that the additional weight burden of the radio-tags themselves may have inhibited capture of food and also made juveniles more vulnerable to goshawks.[393]
Parasitism and bacterial infections
Helminths are also fairly common and may compromise the condition of many owls, if rarely the cause of direct fatalities.[394] Parasites commonly recorded in tawny owls including Leucocytozoon, Haemoproteus, Toxoplasma, Trypanosoma, Centrorhynchus and Tetrameres, with different species of Leucocytozoon present in about 95% of 32 individuals in Great Britain and 57% of study specimens in Italy.[7][395][396][397] Haemoptroteus was the prevalent parasites in France and also quite prominent in birds from Turkey.[398][399] The first records of Trichinella were recorded recently in Europe as well as the similarly rare Eimeria.[400][401] Blood parasitism was more common in England when food supplies were lowered.[402][403] In some cases, parasites are contracted directly from their prey.[404] It was estimated that about 25% of free-living adults in England had some variety of ocular lesions, which may lead to cases of bacterial infections and conjunctiva.[405] Concentrations of Enterobacteriaceae, which can be fatal to humans, are sometimes found in nest boxes.[406]
Status

The tawny owl is a common bird, especially in central Europe.[4] A mid-1990s estimate of the European population was 400,000-800,000 pairs.[7] The tawny owl has a geographical range of at least 10 million km2 (3.8 million mi2) and a large population including an estimated 970,000–2,000,000 individuals in Europe alone with more extensive surveys two decades later.[1][407] Population trends have not been quantified, but there is evidence of an overall increase. This owl is not believed to meet the IUCN Red List criterion of declining more than 30% in ten years or three generations and is therefore evaluated as being of least concern.[1][407] Globally, the population may be estimated anywhere from 1 million to nearly 3 million individuals.[1] 50% of European numbers were in the nations of Germany, France, Poland, Italy and Russia.[7][407] In the UK it is on the RSPB Amber List of Concern.[408] In Great Britain, estimated number there in the 1970s ran up to 100,000 pairs whereas more recent estimate suggest much fewer, at about 20,000 pairs.[407] Although estimates are variable, close study has shown a population reduction of fairly nebulous cause in Britain of around 11% from 1968-1972 to 1988-1991 or of an estimated 6% from 1970 to 2010, utilizing two different surveying methods.[409][410][411] Relative to the loss of other British woodland birds, which has been pronounced, the tawny owl reductions may be considered small to moderate. Among myriad and complex causes for all forest birds in Britain, for the tawny owl it is mentioned that competition for nest sites with introduced eastern gray squirrels (Sciurus carolinensis) may be responsible for some of the reductions.[412] The French population of tawny owls is very large and increased in estimated numbers from 10,000-100,000 in the early 1970s to 50,000-150,000 estimated in the 1990s.[7] 5 digit numbers are estimated in many European countries, including Germany, Italy, Poland, Norway, Sweden, Spain, Greece, Croatia, European Russia and Belarus.[7] It is thought that declines may have occurred in Finland, Estonia, Albania and even Italy.[116] The estimated number of breeding pairs within Denmark using different calculation methods was very variable with different estimates from 4800 to 7400 to 20,500.[413] A large number were estimated in Turkey, perhaps up to an estimated 25,000 pairs, with Syria estimated up to 50 pairs and 250 pairs roughly in Israel.[1][7] While tawny owls may commonly use nest boxes, these are not considered necessary given the general stability of the population.[4] Increased populations pose a risk to smaller owls in areas of sympatry and may hamper conservation efforts of such species.[4][316]
Besides natural causes such as predation and starvation, collision with vehicles, power lines, any other kind of wire collision and other manmade objects is a regular cause of tawny owl mortality.[7][414] Occasionally, the species is exposed to assorted forms of human persecution (from egg-collecting to shootings and poisonings) but it is not nearly as extensively persecuted as larger birds like golden eagles, goshawks and eagle owls. However, earlier in the 20th century, as many as 12% of birds recovered in Scandivania were dead from various forms of persecution.[6][163][415][416] Although they are fairly adaptable to urbanization, there are limits to the tawny owls adaptability, especially due to declines in woodland quality, prey access and human-sourced mortality sources, one of the worst of which is collision with vehicles.[7][417] Of 112 tawny owls re-released in the wild from England's East Winch Wildlife Centre, 17% were subsequently recovered (about half of which were dead), with the most diagnosed cause of mishap being road accidents.[418] Of 57 tawny owls released in Somerset and Hampshire, 28% were recovered dead.[419] However, tawny owls, perhaps due to their liking of wooded rather open areas, are relatively less vulnerable to automobile deaths than some other owls, i.e. of hundreds dead owls on roadsides in Italy, only 4.5% were tawnys, while 41% were little owls and 39% barn owls, while in Germany, 7.4% were tawnys, against 53% barn owls and 28.4% little owls.[420] Variable levels of occular damage, with comparable levels of ocular trauma to humans who are victims of car collisions, were detected in 50 tawny owls in England recovered after surviving car collisions.[421] Damage to the occular area was recorded in 128 of 216 free-living tawnys in southwest England, and in 60.5% of 147 admitted birds the injury were due to road accidents.[422] In the forest of Burgundy, road-based deaths were the main cause of mortality that could be diagnosed.[423] Roadkills of tawny owls were fairly frequent in Portugal, but were not usually in prime habitat and often occurred near the borders of existing territories.[424] However, density was reduced by road development in Portuguese owls, largely due to the fragmentation of habitat, disturbance and noise pollution that may effect intraspecific communication and hunting and increase mortality may come with major roads for owls.[425] Similarly, in Germany, despite the general adaptability to development in tawny owls, locally the noise pollution and increased fragmentation of woods was found to be reducing tawny owl populations.[426] An unclear number of tawny owls may be killed by heavy metal contamination via bioaccumulation as well as via pesticide use.[4][427] Lead levels were difficult to estimate in Spanish owls due to the large number of feathers needed to estimate them, while dead Spanish owls had middling, variable levels of mercury concentrations.[428][429][430] In eggs from Norway, polyfluoroalkyl compounds were studied, in sum toxicological implications were low and several times lower than would be implied by the diversity of compounds found in the eggs.[431] British birds appear to be effected by second-generation anticoagulants, with about 20% of 192 birds showing detectable residues of these. The number is lower than other raptors analyzed here such as common kestrel, barn owl, common buzzard and red kite and the number of tawnys did not perceptibly decline due to this.[432] In Sweden and perhaps Germany, the level of PFAS and mercury were fairly low, comparable to common kestrel and much lower than the levels in ospreys (Pandion haliaetus).[433][369][434]
In culture

The tawny owl, like its relatives, has often been seen as an omen of bad luck; William Shakespeare used it as such in Julius Caesar (Act 1 Scene 3): "And yesterday the bird of night did sit/ Even at noon-day upon the market-place/ Hooting and shrieking." John Ruskin is quoted as saying "Whatever wise people may say of them, I at least have found the owl's cry always prophetic of mischief to me".[435]
Wordsworth described the technique for calling an owl in his poem There was a Boy.[436]
And there, with fingers interwoven, both hands
Pressed closely palm to palm and to his mouth
Uplifted, he, as through an instrument,
Blew mimic hootings to the silent owls,
That they might answer him.—And they would shout
Across the watery vale, and shout again,
Responsive to his call,—with quivering peals,
And long halloos, and screams, and echoes loud
Redoubled and redoubled; concourse wild
Of jocund din!
References
- BirdLife International (2016). "Strix aluco". IUCN Red List of Threatened Species. 2016: e.T22725469A86871093. doi:10.2305/IUCN.UK.2016-3.RLTS.T22725469A86871093.en.
- For Syrnium aluco see for instance: Powys, Baron Lilford, Thomas Littleton; Salvin, Osbert; Newton, Alfred; Keulemans, John Gerrard (1885). Coloured figures of the birds of the British Islands. 1. London: R.H. Porter. pp. 87f. OCLC 1029665771. Retrieved 2020-05-15.
- Sclater, P. L. (1879). Remarks on the Nomenclature of the British Owls, and on the Arrangement of the Order Striges. Ibis, 21(3), 346-352.
- König, Claus; Weick, Friedhelm (2008). Owls of the World (2nd ed.). London: Christopher Helm. ISBN 9781408108840.
- Weick, Friedhelm (2007). Owls (Strigiformes): Annotated and Illustrated Checklist. Springer. ISBN 978-3-540-39567-6.
- Voous, Karel H.; Cameron, Ad (illustrator) (1988). Owls of the Northern Hemisphere. London, Collins. pp. 209–219. ISBN 978-0-00-219493-8.
- Galeotti, P. (2001). Strix aluco Tawny Owl. Birds of Western Palearctic. Update, 3(1), 43-77.
- Adanez, A. V., & Hontanilla, C. T. M. (1983). Alimentación del cárabo (Strix aluco L. 1758) en España. Alytes, 1, 291-306.
- Martin, J. (1990). Amphibians and reptiles as prey of birds in southwestern Europe. Smithsonian Herpetological Information Service.
- Hume, R. (1991). Owls of the world. Running Press, Philadelphia.
- Koning, F. J., Koning, H. J., & Baeyens, G. (2009). Long-term study on interactions between Tawny Owls Strix aluco, Jackdaws Corvus monedula and Northern Goshawks Accipiter gentilis. Ardea, 97(4), 453-457.
- Galeotti, P. (1990). Territorial behaviour and habitat selection in an urban population of the tawny owl Strix aluco L. Italian Journal of Zoology, 57(1), 59-66.
- Mikkola, H. (2000). General public knowledge of owls in Finland. Buteo, 11, 5-18.
- Mullarney, Killian; Svensson, Lars; Zetterstrom, Dan; Grant, Peter J. (1999). Collins Bird Guide. London: HarperCollins. p. 206. ISBN 978-0-00-219728-1.
- Brown, R.H. (1936). Tawny Owl taking prey during the day. British Birds 30, 173–4.
- Neuhaus, W., Bretting, H., & Schweizer, B. (1973). Morphologische und funktionelle Untersuchungen über den „lautlosen" Flug der Eulen (Strix aluco) im Vergleich zum Flug der Enten (Anas platyrhynchos). Biologisches Zentralblatt, 92, 495-512.
- RSPB Handbook of British Birds (2014). ISBN 978-1-4729-0647-2.
- Petty, S. J. (1994). Moult in Tawny Owls Strix aluco in relation to food supply and reproductive success. Raptor Conservation Today, 521-530.
- Petty, S. J. (1992). A guide to age determination of Tawny Owl Strix aluco. The Ecology and Conservation of European Owls, 89-91.
- Emaresi, G., Ducrest, A. L., Bize, P., Richter, H., Simon, C., & Roulin, A. (2013). Pleiotropy in the melanocortin system: expression levels of this system are associated with melanogenesis and pigmentation in the tawny owl (Strix aluco). Molecular ecology, 22(19), 4915-4930.
- Koskenpato, K., Ahola, K., Karstinen, T., & Karell, P. (2016). Is the denser contour feather structure in pale grey than in pheomelanic brown tawny owls Strix aluco an adaptation to cold environments? Journal of Avian Biology, 47(1), 1-6.
- Emaresi, G., Bize, P., Gasparini, J., Piault, R., & Roulin, A. (2011). Plumage polymorphism in melanin-based coloration: a case study in the tawny owl Strix aluco. Ecology and conservation of european forest-dwelling raptors, 242-252.
- Brommer, Jon E.; Kari, Ahola; Karstinen, Teuvo (2005). "The colour of fitness: plumage coloration and lifetime reproductive success in the tawny owl". Proceedings of the Royal Society of London B: Biological Sciences. 272 (1566): 935–940. doi:10.1098/rspb.2005.3052. PMC 1564093. PMID 16024349.
- Galeotti, Paolo; Sacchi, Roberto (2003). "Differential parasitaemia in the Tawny Owls (Strix aluco): effects of colour morph and habitat". Journal of Zoology. 261: 91–99. doi:10.1017/S0952836903003960.
- Gryz, J., & Krauze-Gryz, D. (2013). Plumage colour polymorphism among central Poland's tawny owls Strix aluco Linnaeus, 1758. Zoology and Ecology, 23(1), 58-60.
- "Tawny Owl Strix aluco [Linnaeus, 1758]". BirdFacts. British Trust for Ornithology (BTO). Retrieved 31 May 2008.
- Mueller, H. C. (1986). The evolution of reversed sexual dimorphism in owls: an empirical analysis of possible selective factors. The Wilson Bulletin, 387-406.
- Lundberg, A. (1986). Adaptive advantages of reversed sexual size dimorphism in European owls. Ornis Scandinavica, 133-140.
- Eurasian Tawny Owl – Strix aluco. The Owl Pages
- Sunde, P., Bølstad, M. S., & Møller, J. D. (2003). Reversed sexual dimorphism in tawny owls, Strix aluco, correlates with duty division in breeding effort. Oikos, 101(2), 265-278.
- Martinez, J.A., Zuberogoitia, I. & Alonso, R. (2002). Guía para la determinación de la edad y el sexo en las estrigiformes ibéricas- CÁRABO COMÚN TAWNY OWL Strix aluco. Monticola: 95-102.
- Owls of the World: A Photographic Guide by Mikkola, H. Firefly Books (2012), ISBN 9781770851368
- Holt, D.W., Berkley, R., Deppe, C., Enríquez Rocha, P., Petersen, J.L., Rangel Salazar, J.L., Segars, K.P., Wood, K.L. & Marks, J.S. (2019). Typical Owls. In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
- CRC Handbook of Avian Body Masses by John B. Dunning Jr. (Editor). CRC Press (1992), ISBN 978-0-8493-4258-5.
- Baudvin, H., & Dessolin, J. L. (1992). Analyse de la morphométrie de la chouette hulotte Strix aluco en Bourgogne. Alauda, 60(2), 93-104.
- Solonen, T. (2011). Effects of variable food supply on the body condition of breeding Tawny Owls Strix aluco in southern Finland. ISRN Zoology, 2011.
- Hardy, A. R., Hirons, G. J. M., Stanley, P. I., & Huson, L. W. (1981). Sexual dimorphism in size of tawny owls (Strix aluco): a method for sexing in field studies. Ardea, 69, 181-184.
- Burton, Robert (1985). Bird Behaviour. London: Granada Publishing. pp. 44–48. ISBN 978-0-246-12440-1.
- Norberg, R. Å. (1977). Occurrence and independent evolution of bilateral ear asymmetry in owls and implications on owl taxonomy. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 280(973), 375-408.
- Voous, K. H. (1964). Wood owls of the genera Strix and Ciccaba. Zoologische Mededelingen, 39(46), 471-478.
- Schwartzkopff, J. (1963). Morphological and physiological properties of the auditory system in birds. Proc. XIII Inter. Ornithol. Congr, 1059-1068.
- Galeotti, P., & Pavan, G. (1991). Individual recognition of male Tawny Owls (Strix aluco) using spectrograms of their territorial calls. Ethology Ecology & Evolution, 3(2), 113-126.
- Andersen T. (1961). En nordsjaellansk Natugle-bestand (Strix aluco L.) i yngletiden. [A population of tawny owl (Strix aluco) in Northern Zealand, studied in the breeding season]. Dansk Or- nithologisk Forenings Tidsskrift, 55: 1-5.
- Southern, H.N. (1970). The Natural Control of a Population of Tawny Owl (Strix aluco). The Journal of Zoology, 162: 197-285.
- Hirons, G.J.R. (1976). A population study of the Tawny Owl Strix aluco) and its main prey species in woodland. D. Phil Thesis, University of Oxford.
- Wendland, V. (1963). Fünfjährige Beobachtungen an einer Population des Waldkauzes (Strix aluco) im Berliner Grunewald. Journal für Ornithologie, 104(1), 23-57.
- Redpath, S. M. (1994). "Censusing Tawny Owls Strix aluco by the use of imitation calls". Bird Study. 41 (3): 192–198. doi:10.1080/00063659409477219. Retrieved 11 December 2019.
- Appleby B. M., Yamaguchi, N., Johnson, P. J., & Macdonald, D. W. (1999). Sex‐specific territorial responses in Tawny Owls Strix aluco. Ibis, 141(1), 91-99.
- Galeotti, P., & Pavan, G. (1993). Differential responses of territorial Tawny Owls Strix aluco to the hooting of neighbours and strangers. Ibis, 135(3), 300-304.
- Zuberogoitia, I.; Burgos, G.; González‐Oreja, J.A.; Morant, J.; Martínez, J.E.; Albizua, J.Z. (2019). "Factors affecting spontaneous vocal activity of Tawny Owls Strix aluco and implications for surveying large areas". Ibis. 161 (3): 495–503. doi:10.1111/ibi.12684.
- Redpath, Stephen M.; Appleby, Bridget M.; Petty, Steve J. (2000). "Do male hoots betray parasite loads in Tawny Owls?". Journal of Avian Biology. 31 (4): 457–462. doi:10.1034/j.1600-048X.2000.310404.x.
- Galeotti, P. (1998). Correlates of hoot rate and structure in male Tawny Owls Strix aluco: implications for male rivalry and female mate choice. Journal of Avian Biology, 29, 25-32.
- Galeotti, P. (1994). Oral communication: Vocal and behavioural repertoire and intensity of aggression of Tawny owls (Strix aluco) in response to playback of conspecific calls.
- Appleby, B. M., & Redpath, S. M. (1997). Variation in the male territorial hoot of the Tawny Owl Strix aluco in three English populations. Ibis, 139(1), 152-158.
- Lengagne, T., & Slater, P. J. (2002). The effects of rain on acoustic communication: tawny owls have good reason for calling less in wet weather. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1505), 2121-2125.
- Appleby, B. M., & Redpath, S. M. (1997). Indicators of male quality in the hoots of tawny owls (Strix aluco). Journal of Raptor Research, 31, 65-70.
- Galeotti, P. R., Appleby, B. M., & Redpath, S. M. (1996). Macro and microgeographical variations in the 'hoot' of Italian and English tawny owls (Strix aluco). Italian Journal of Zoology, 63(1), 57-64.
- Shekhovtsov, S. M., & Sharikov, A. V. (2015). Individual and geographical variation in the territorial calls of Tawny Owls Strix aluco in Eastern Europe. Ardeola, 62(2), 299-311.
- Spencer, K.G. (1953) Bird Notes News, 25, 141–4.
- Harrison, R. (1947). Gurgling note of Tawny Owl. British Birds, 40: 181.
- Scherzinger, W. (1980). Zur Ethologie der Fortpflanzung und Jugendentwicklung des Habichtkauzes (Strix uralensis) mit Vergleichen zum Waldkauz (Strix aluco). Bonn. Zool. Mongr, 15.
- Wahlstedt, J. (1959). Ugglornas spelvanor. Fauna Flora, 45, 81-112.
- Churchill, W.J. (1939). Ool. Rec., 19: 35–36.
- Stülcken, K. (1961). Beobachtungen an einem Waldkauzpärchen. Falke, 8, 39-45.
- Based on Güntürkün, Onur, "Structure and functions of the eye" in Sturkie, P. D. (1998). Sturkie's Avian Physiology. 5th Edition. Academic Press, San Diego. pp. 1–18. ISBN 978-0-12-747605-6.
- Brooke, M. D. L., Hanley, S., & Laughlin, S. B. (1999). The scaling of eye size with body mass in birds. Proceedings of the Royal Society of London. Series B: Biological Sciences, 266(1417), 405-412.
- Hecht, Selig; Pirenne, Maurice Henri (1940). "The sensibility of the nocturnal long-eared owl in the spectrum" (Automatic PDF download). Journal of General Physiology. 23 (6): 709–717. doi:10.1085/jgp.23.6.709. PMC 2237955. PMID 19873186.
- Martin, Graham R. (August 1977). "Absolute visual threshold and scotopic spectral sensitivity in the tawny owl Strix aluco". Nature. 268 (5621): 636–638. Bibcode:1977Natur.268..636M. doi:10.1038/268636a0. PMID 895859. S2CID 4184444.
- Martin, G. R., & Gordon, I. E. (1974). Visual acuity in the tawny owl (Strix aluco). Vision Research, 14(12), 1393-1397.
- Martin, G. R. (1974). Clor vision in the tawny owl (Strix aluco). Journal of comparative and physiological psychology, 86(1), 133.
- Martin, G. R. (1973). Operant responding and stimulus control in tawny owls (Strix aluco). Journal of comparative and physiological psychology, 85(2), 346.
- Ferens, B. (1947). On the ability of colour-discrimination of the tawny owl (Strix aluco aluco L.). Bull. Int. Acad. Pol. Sci. Let. Cl. Med.(Series B, II), 300, 337.
- Bowmaker, J. K., & Martin, G. R. (1978). Visual pigments and colour vision in a nocturnal bird, Strix aluco (tawny owl). Vision Research, 18(9), 1125-1130.
- Sinclair, Sandra (1985). How Animals See: Other Visions of Our World. Beckenham, Kent: Croom Helm. pp. 88–100. ISBN 978-0-7099-3336-6.
- Martin, G. R. (1982). An owl's eye: Schematic optics and visual performance in Strix aluco L. Journal of comparative physiology, 145(3), 341-349.
- Martin, G. R. (1984). The visual fields of the tawny owl, Strix aluco L. Vision research, 24(12), 1739-1751.
- Martin, G. R. (1986). Sensory capacities and the nocturnal habit of owls (Strigiformes). Ibis, 128(2), 266-277.
- Crossley, R., & Couzens, D. (2014). The Crossley ID Guide: Britain & Ireland. Crossley Books, Princeton University Press.
- Peterson, R. T., Mountfort, G., & Hollom, P. A. D. (2001). A field guide to the birds of Britain and Europe (Vol. 8). Houghton Mifflin Harcourt.
- Mikkola, H. (1983). Owls of Europe. T. & AD Poyser.
- Linnaeus, C (1758). Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata (in Latin). Holmiae. (Laurentii Salvii). p. 93.
S. capite laevi, corpore ferrugineo, iridíbus atris, remi-gibus primoribus serratís.
- Allen, J. A. (1908). The case of Strix vs. Aluco. The Auk, 25(3), 288-291.
- Sibley, C. G., & Ahlquist, J. E. (1990). Phylogeny and classification of birds: a study in molecular evolution. Yale University Press.
- Gill, Frank; Donsker, David, eds. (2019). "Owls". World Bird List Version 9.1. International Ornithologists' Union. Retrieved 2 April 2019.
- Wink, M., El-Sayed, A. A., Sauer-Gürth, H., & Gonzalez, J. (2009). Molecular phylogeny of owls (Strigiformes) inferred from DNA sequences of the mitochondrial cytochrome b and the nuclear RAG-1 gene. Ardea, 97(4), 581-592.
- Mlíkovský, Jirí (2002): Cenozoic Birds of the World, Part 1: Europe. Ninox Press, Prague.
- Lee, M. Y., Lee, S. M., Jeon, H. S., Lee, S. H., Park, J. Y., & An, J. (2018). Complete mitochondrial genome of the Northern Long-eared Owl (Asio otus Linnaeus, 1758) determined using next-generation sequencing. Mitochondrial DNA Part B, 3(2), 494-495.
- van der Weyden, W. J. (1975). Scops and screech owls: vocal evidence for a basic subdivision in the genus Otus (Strigidae). Ardea, 63, 65-77.
- Salomonsen, F. (1931). Beretning om en Rejse til Færøerne. Dansk Orn. Foren. Tidsskr, 25, 3-37.
- Jánossy D. (1972). Die mittelpleistozäne Vogelfauna der Stránská skála. – In: Musil R. (ed.): Stránská skála I. – Anthropos (Brno) 20: 35-64.
- Jánossy D. (1976). Die Felsnische Tarkő und die Vertebratenfauna ihrer Ausfüllung. Karsztés Barlangkutatás 8: 3-106.
- Jánossy, D. 1978. Új finomrétegtani szint Magyarország pleisztocén őslénytani sorozatában [A new fine stratigrafic level in Paleontological series at Hungarian Pleistocene]. Földrajzi Közlemények 26(1–3): 161–174.
- Mourer-Chauvire, C. (1975). Faunes d'oiseaux du Pléistocene de France: systématique, évolution et adaptation, interprétation paléoclimatique. Geobios, 8(5), 333-IN11.
- Becker, C., & Pieper, H. (1982). Zum Nachweis des Habichtkauzes Strix uralensis in einer neolithischen Seeufersiedlung der Schweiz. Der Ornithologische Beobachter, 79, 159-162.
- Scherzinger, W. (1983). Beobachtungen an Waldkauz-Habichtskauz-Hybriden:(Strix aluco x Strix uralensis).
- Heidrich, P., & Wink, M. (1994). Tawny owl (Strix aluco) and Hume's tawny owl (Strix butleri) are distinct species: evidence from nucleotide sequences of the cytochrome b gene. Zeitschrift für Naturforschung C, 49(3-4), 230-234.
- Kirwan, G. M., Schweizer, M., & Copete, J. L. (2015). Multiple lines of evidence confirm that Hume's Owl Strix butleri (AO Hume, 1878) is two species, with description of an unnamed species (Aves: Non-Passeriformes: Strigidae). Zootaxa, 3904(1), 28-50.
- Robb, M. S., Sangster, G., Aliabadian, M., van den Berg, A. B., Constantine, M., Irestedt, M., Khani, A., Musavi, S.B., Nunes, J.M.G., Willson, M.S. & Walsh, A. J. (2016). The rediscovery of Strix butleri (Hume, 1878) in Oman and Iran, with molecular resolution of the identity of Strix omanensis Robb, van den Berg and Constantine, 2013. Avian Research, 7(1), 7.
- Del Hoyo, J., Collar, N. J., Christie, D. A., Elliott, A., & Fishpool, L. D. C. (2014). HBW and BirdLife International Illustrated Checklist of the Birds of the World: non-passerines (Vol. 1). Barcelona: Lynx Edicions.
- Sibley, C. G., & Monroe Jr, B. L. (1993). A world checklist of birds. Ann Arbor, MI: Edwards Brothers Inc.
- Doña, J., Ruiz-Ruano, F. J., & Jovani, R. (2016). DNA barcoding of Iberian Peninsula and North Africa Tawny Owls Strix aluco suggests the Strait of Gibraltar as an important barrier for phylogeography. Mitochondrial DNA Part A, 27(6), 4475-4478.
- Dov, A.B., Atar, A., Baruchi, E, Levi, K. & Sapir, N. (2017). Breeding biology of Desert Owl in Israel. Dutch Birding, 39, 285-307.
- Marcot, B. G. (1995). Owls of old forests of the world. Gen. Tech. Rep. PNW-GTR-343. Portland, OR: US Department of Agriculture, Forest Service, Pacific Northwest Research Station. 64 p, 343.
- "Tawny Owl Strix aluco". Owl Information. World Owl Trust. Archived from the original on 13 June 2008. Retrieved 7 November 2017.
- Brito, P. H. (2007). Contrasting patterns of mitochondrial and microsatellite genetic structure among Western European populations of tawny owls (Strix aluco). Molecular Ecology, 16(16), 3423-3437.
- Scully, J. (1881). A Contribution to the Ornithology of Gilgit. Ibis, 23(3), 415-453.
- Grewal, B., & Pfister, O. (1998). A photographic guide to birds of the Himalayas. New Holland.
- Blanford, W. T. (1894). Notes on the Indian Owls. Ibis, 36(4), 524-531.
- Zarudny, N. 1905. [Itinerary of the voyage to western Iran in the year 1903–1904]. Ezhegodnik' Zoologicheskago Muzeya Imperatorskoi Akademii Nauk 9.
- Zarudny, N.A. (1911) Verzeichnis der Vögel Persiens. Journal für Ornithologie, 59: 185-241.
- Dementiev, G. P., Gladkov, N. A., Ptushenko, E. S., Spangenberg, E. P., & Sudilovskaya, A. M. (1966). Birds of the Soviet Union, vol. 1. Israel Program for Scientific Translations, Jerusalem.
- Bergmann, Carl (1847). "Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse". Göttinger Studien (in German). 3 (1): 595–708.
- Lowen, J. & Bocos, C. (2019). Birds of Spain. Helm.
- Heinzel, H., Fitter, R. S. R., & Parslow, J. (1972). The Birds of Britain and Europe with North Africa and the Middle East. London: Collins.
- Merikallio, E. (1958). Finnish birds: their distribution and numbers. Soc. pro fauna et flora Fennica.
- Snow, David (1998). Perrins, Christopher M. (ed.). The Birds of the Western Palearctic concise edition (two volumes). Oxford: Oxford University Press. pp. 907–910. ISBN 978-0-19-854099-1.
- Seamons, D. (1997). Field Guide to the Birds of the Middle East. Reference Reviews.
- Ayé, R., Schweizer, M., & Roth, T. (2012). Birds of Central Asia. Bloomsbury Publishing.
- Ali, S., & Ripley, S. D. (1983). Handbook of the Birds of India and Pakistan: Together with Those of Bangladesh, Nepal, Bhutan and Sri Lanka. Oxford University Press.
- Neufeldt, I. A. (1958). On avifauna of the Southern Karelia. Proceedings of the Zoological Institute Academy of Sciences of the USSR, 25, 183-254.
- Cesaris, C. (1988). Popolazioni di Allocco Strix aluco e di Civetta Athene noctua in un'area del Parco Lombardo della Valle del Ticino. Avocetta, 12, 115-118.
- Bernis, F. (1956). Nota preliminar sobre aves de Asturias y Galicia. Ardeola, 3(1), 31-49.
- Kajtoch, Ł., Żmihorski, M., & Wieczorek, P. (2015). Habitat displacement effect between two competing owl species in fragmented forests. Population ecology, 57(3), 517-527.
- Arslangündoğdu, Z., Beşkardeş, V., Smith, L., & Yüksel, U. (2013). The Tawny Owl (Strix aluco L., 1758) Population in Belgrad Forest, Istanbul–Turkey. İstanbul Üniversitesi Orman Fakültesi Dergisi, 63(1), 11-17.
- Sharikov, A. V., & Shekhovtsov, S. M. (2013). Seasonal and daily vocal activity of the tawny owl (Strix aluco) and the pygmy owl (Glaucidium passerinum) in Moscow Region. Zoologicheskii Zhurnal, 92(1), 68-76.
- Fröhlich, A.; Ciach, M. (2018). "Noise pollution and decreased size of wooded areas reduces the probability of occurrence of Tawny Owl Strix aluco". Ibis. 160 (3): 634–646. doi:10.1111/ibi.12554.
- Rumbutis, S., Vaitkuvienė, D., Grašytė, G., Dagys, M., Dementavičius, D., & Treinys, R. (2017). Adaptive habitat preferences in the Tawny Owl Strix aluco. Bird study, 64(3), 421-430.
- Redpath, S. M. (1995). Habitat fragmentation and the individual: tawny owls Strix aluco in woodland patches. Journal of Animal Ecology, 652-661.
- Sunde, P., & M. Redpath, S. (2006). Combining information from range use and habitat selection: sex‐specific spatial responses to habitat fragmentation in tawny owls Strix aluco. Ecography, 29(2), 152-158.
- Bolboaca, L. E., Baltag, E. S., Pocora, V., & Ion, C. (2013). Habitat selectivity of sympatric Tawny Owl (Strix aluco) and Ural Owl (Strix uralensis) in hill forests from north-eastern Romania. Analele Științifice ale Universității „Alexandru Ioan Cuza" din Iași, s. Biologie animală, 59, 69-76.
- Salvati, L., & Ranazzi, L. (2002). Changes in density and territory size of the Tawny Owl Strix aluco along an altitude gradient: the effect of forest types and wood cover. Acta zoologica cracoviensia, 45(2), 237-243.
- Baxter, E. V., & Rintoul, L. J. (1953). The birds of Scotland: Their history, distribution, and migration (Vol. 2). Oliver and Boyd.
- Glutz von Blotzheim, U. N., Bauer, K. M., & Bezzel, E. (1980). Handbuch der vögel mitteleuropas. Aula, Wiesbaden.
- Cucco, M. & Beltramino, M. (1998). Allocco Strix aluco . In Mingozzi, T., Boano, G., Pulcher, C. & Maffei, G. (eds), Atlante degli Uccelli nidificanti in Piemonte e Val d'Aosta 1980–1984, p. 192. Monografia VIII, 87–91.Mus. reg. Sci. nat. Torino, Torino.
- Beven, G. (1965). The food of Tawny Owls in London. London Bird Reports, 29: 56–72.
- Korpimäki, E. (1986). Niche relationships and life-history tactics of three sympatric Strix owl species in Finland. Ornis Scandinavica, 126-132.
- Wille, H. G. (1972). Ergebnisse einer mehrjährigen Studie an einer Population des Waldkauzes (Strix aluco) in West-Berlin. Orn. Min. 24: 3, 7.
- Redpath, S. M. (1995). Impact of habitat fragmentation on activity and hunting behavior in the tawny owl, Strix aluco. Behavioral Ecology, 6(4), 410-413.
- Šušić, V. T., & Kovačević, R. M. (1973). Sleep patterns in the owl Strix aluco. Physiology & behavior, 11(3), 313-317.
- Sunde, P., Bølstad, M. S., & Desfor, K. B. (2003). Diurnal exposure as a risk sensitive behaviour in tawny owls Strix aluco? Journal of Avian Biology, 34(4), 409-418.
- Bech, C., & Præsteng, K. E. (2004). Thermoregulatory use of heat increment of feeding in the tawny owl (Strix aluco). Journal of Thermal Biology, 29(7-8), 649-654.
- Basecke, K. (1942). Zur Paarbildung und Revierbehauptung beim Walkauz. Beitr. Fortpfl. Biol. Vogel, 18: 143-144.
- Sunde, P., & Bølstad, M. S. (2004). A telemetry study of the social organization of a tawny owl (Strix aluco) population. Journal of Zoology, 263(1), 65-76.
- Hirons, G. J. M. (1982). The effects of fluctuations in rodent numbers on breeding success in the Tawny Owl Strix aluco. Mammal Review, 12(4), 155-157.
- Hardy, A. R. (1992). Habitat use by farmland Tawny Owls Strix aluco. The ecology and conservation of European owls, 55-63.
- Zuberogoitia, I., & Martínez, J. A. (2000). Methods for surveying Tawny Owl Strix aluco populations in large areas. Biota, 1(2), 79-88.
- Sánchez-Zapata, J. A., & Calvo, J. F. (1999). Rocks and trees: habitat response of Tawny Owls Strix aluco in semiarid landscapes. Ornis Fennica, 76(2), 79-87.
- Ziesemer, F. (1979). Zur methodik von bestandsaufnahmen am Waldkauz (Strix aluco) mit Hilfe von Klangattrapen. Corax, 7: 106-108.
- Wiącek, J., Polak, M., & Grzywaczewski, G. (2010). The role of forest age, habitat quality, food resources and weather conditions for Tawny Owl Strix aluco populations. Polish Journal of Environmental Studies, 19(5), 1039-1043.
- Jabłoński, P. (1991). Rozmieszczenie puszczyka Strbc aluco w Warszawie. Acta Ornithologica, 26(1).
- Cios, S., & Grzywaczewski, G. (2013). Importance of selected factors influencing the size of tawny owl Strix aluco territories in the forests of Lublin region. Sylwan, 157(5), 348-357.
- Galeotti, P. (1994). Patterns of territory size and defence level in rural and urban tawny owl (Strix aluco) populations. Journal of Zoology, 234(4), 641-658.
- Salvati, L., Manganaro, A, & Ranazzi, L. (2002). Wood quality and the Tawny Owl Strix aluco in different forest types of central Italy. Ornis Svecica, 12, 47-51.
- Ranazzi, L., Manganaro, A., Pucci, L., & Salvati, L. (2001). High densities of the Tawny Owl (Strix aluco) in mature deciduous forests of Latium (central Italy). Buteo, 12: 111-118.
- Ranazzi, L., Manganaro, A., Ranazzi, R., & Salvati, L. (2000). Woodland cover and Tawny Owl Strix aluco density in a Mediterranean urban area. Biota, 1, 83-92.
- Ranazzi, L., Manganaro, A., & Salvati, L. (2002). Density fluctuations in an urban population of Tawny Owl Strix aluco: a long-term study in Rome, Italy. Ornis Svecica, 12, 63-67.
- Delmée, E., Dachy, P., & Simon, P. (1978). Fifteen years of observations on the reproductions of a forest population of a Tawny Owls (Strix aluco). Gerfaut, 68(4), 590-650.
- Dambiermont, J. L., Francotte, J. P., & Collette, P. (1967). Notes sur la nidification des Hulottes (Strix aluco) en nichoirs. Aves, 4, 31-47.
- Vrezec, A., & Saveljić, D. (2006). Breeding density of Tawny Owl Strix aluco territories in montane forests of Mountain Bjelasica (Montenegro). Ciconia, 14, 41-47.
- Bolboacă, L. E., Artem E., & Amarghioalei, V. (2015). Breeding densities of Tawny Owl (Strix aluco) in eastern Moldova region (Romania). BIOLOGIE ANIMALĂ.
- Nilsson, I. N. (1977). Hunting methods and habitat utilization of two Tawny Owls (Strix aluco L.). Fauna och Flora, 72, 156-163.
- Sunde, P., Overskaug, K., Bolstad, J. P., & Øien, I. J. (2001). Living at the limit: ecology and behaviour of Tawny Owls Strix aluco in a northern edge population in central Norway. Ardea, 89(3), 495-508.
- Olsson, V. (1958). Dispersal migration, longevity and death causes of Strix aluca, Buteo buteo, Ardea cinerea and Larus argentatus: a study based on recoveries of birds ringed in Fenno-Scandia. Almqvist & Wiksell.
- Hardy, A.R. (1977). Hunting ranges and feeding ecology of owls in farmland. Ph.D. thesis, Aberdeen University.
- Bennett, J. A., & Routh, A. D. (2000). Post-release survival of hand-reared tawny owls (Strix aluco). Animal Welfare, 9(3), 317-321.
- Bernhoft-Osa, A. (1973). Hvor tungt bytte klarer rovfuglene å fly avsted med. Sterna, 12, 73-83.
- Meinerzhagen, R. (1959). Pirates and Predators: The piratical and predatory habits of birds. Oliver & Boyd
- Nilsson, I. N. (1978). Hunting in flight by Tawny Owls Strix aluco. Ibis, 120(4), 528-531.
- Uttendorfer, O. (1939). Die Ernahrung der Deutschen Raub- vogel und Eulen und ihre Bedeutung in der Heimischer. Natur. Verl. Neumann, Neudamm.
- Fitter, R. S. R. (1949). London's birds. Collins.
- Ruthke, P. (1935). Uber das Rutteln des Walkauzes. Beitr. Fortpfl. Biol. Vogel, 12: 79.
- Mather, J. (1979). Tawny Owl taking Juvenile Sand Martin in Flight. British Birds, 72(11), 552-552.
- Snow, D. W. (1958). A study of blackbirds. British Museum of Natural.
- Macdonald, D. W. (1976). Nocturnal observations of tawny owls Strix aluco preying upon earthworms. Ibis, 118(4), 579-580.
- Macdonald, D. W. (1983). Predation on earthworms by terrestrial vertebrates. In Earthworm ecology (pp. 393-414). Springer, Dordrecht.
- Schnurre, O. (1934). Ernährung und Jagdbeute des Waldkauzes im Berliner Tiergarten. Beitr. Fortpflanzungsbiol. Vög, 10, 206-213.
- Schneider, W. (1979). Zur mannigfaltigen Fangmethode des Wadkauzes. Beitr. Vogelkd, 25: 364.
- Robert, E.L. (1944). Tawny Owl taking carrion. British Birds, 38: 56.
- Gruzdev, L.V. & Likhachev, G.N. (1960). Contribution to feeding habits of Strix aluco in the Tula Zaseki. Zoologicheskii Zhurnal 39: 624-627 (in Russian with English summary).
- Vásárhelyi, I. (1967). Aquila, 73: 196.
- Kochan, W. (1979). Acta Zool. Cracovia, 23: 213–46.
- Raber, H. (1950). Animal Behaviour, 2.
- Thiollay, J. M. (1963). Les pelotes de quelques rapaces. Nos oiseaux, 26(289-290), 124-131.
- Bütikofer, E (1909). Der Waldkauz. Orn. Beob. 7: 37-40.
- de Brichambaut, J.P. (1978). Mode de dépouillement de mammifères moyens par deux rapaces. Alauda, 46 : 271: 27.
- Brown, Roy; Ferguson, John; Lawrence, Michael; Lees, David (1987). Tracks and Signs of the Birds of Britain and Europe (Helm Identification Guides). Christopher Helm. p. 86. ISBN 978-0-7470-0201-7.
- Cramp, S.; Simmons, K.E.L. (1985). Birds of the Western Palearctic. Vol. 2. Oxford: Oxford University Press.
- Obuch, J. (2011). Spatial and temporal diversity of the diet of the tawny owl (Strix aluco). Slovak Raptor Journal, 5, 1-120.
- Capizzi, D. (2000). Diet shifts of the tawny owl Strix aluco in central and northern Italy. Italian Journal of Zoology, 67(1), 73-79.
- Räber, H. (1949). Das Verhalten gefangener Waldohreulen (Asio otus otus) und Waldkäuze (Strix aluco aluco) zur Beute. Behaviour, 1-95.
- Marti, C. D., Korpimäki, E., & Jaksić, F. M. (1993). Trophic structure of raptor communities: a three-continent comparison and synthesis. In Current ornithology (pp. 47-137). Springer, Boston, MA.
- Herrera, C. M., & Hiraldo, F. (1976). Food-niche and trophic relationships among European owls. Ornis Scand, 7(1), 29.
- Koning, F.J. (1982). Aantekeningen over de Verdeling van de Ruimte en de Voedeselbronnen onden de Roofvogels en Uilen in Onze Duinen. Graspieper, 2: 43-53.
- Marchesi, L., Sergio, F., & Pedrini, P. (2006). Implications of temporal changes in forest dynamics on density, nest-site selection, diet and productivity of Tawny Owls Strix aluco in the Alps. Bird Study, 53(3), 310-318.
- Balciauskiene, L. (2005). Analysis of Tawny owl (Strix aluco) food remains as a tool for long–term monitoring of small mammals. Acta Zool. Lituanica, 15, 85-89.
- Yatsiuk, Y., & Filatova, Y. (2017). Seasonal changes in Tawny Owl (Strix aluco) diet in an oak forest in Eastern Ukraine. Turkish Journal of Zoology, 41(1), 130-137.
- Eppley, T. M., & Donati, G. (2019). Cathemeral. Encyclopedia of Animal Cognition and Behavior, 1-4.
- Southern, H. N., & Lowe, V. P. W. (1982). Predation by tawny owls (Strix aluco) on bank voles (Clethrionomys glareolus) and wood mice (Apodemus sylvaticus). Journal of Zoology, 198(1), 83-102.
- Flowerdew, J. R., & Ellwood, S. A. (2001). Impacts of woodland deer on small mammal ecology. Forestry, 74(3), 277-287.
- Żmihorski, M., Balčiauskienė, L., & Romanowski, J. (2008). Small mammals in the diet of the Tawny owl (Strix aluco) in Central European lowland. Pol. J. Ecol, 56(4), 693-700.
- Lagerström, M., & Häkkinen, I. (1978). Uneven sex ratio of voles in the food of Aegolius funereus and Strix aluco. Ornis Fennica, 55, 149-153.
- Balčiauskas, L., & Balčiauskienė, L. (2014). Selection by size of the yellow-necked mice (Apodemus flavicollis) by breeding Tawny Owl (Strix aluco). North-Western Journal of Zoology, 10(2).
- Sunde, P., Forsom, H. M., Al-Sabi, M. N. S., & Overskaug, K. (2012). Selective predation of tawny owls (Strix aluco) on yellow-necked mice (Apodemus flavicollis) and bank voles (Myodes glareolus). In Annales Zoologici Fennici (Vol. 49, No. 5, pp. 321-331). Finnish Zoological and Botanical Publishing Board.
- Balciauskas, L., & Balciauskiene, L. (2014). Selective predation on common voles by Tawny Owls and Long-eared Owls in winter and spring. Turkish Journal of Zoology, 38(2), 242-249.
- Erlinge, S. (1987). Predation and noncyclicity in a microtine population in southern Sweden. Oikos, 347-352.
- Wendland, V. (1984). The influence of prey fluctuations on the breeding success of the Tawny Owl Strix aluco. Ibis, 126(3), 284-295.
- Petty, S. J. (1992). Ecology of the tawny owl Strix aluco in the spruce forests of Northumberland and Argyll (Doctoral dissertation, The Open University).
- Petty, S. J., & Fawkes, B. L. (1997). Clutch size variation in Tawny Owls (Strix aluco) from adjacent valley systems: can this be used as a surrogate to investigate temporal and spatial variations in vole density? In In: Duncan, James R.; Johnson, David H.; Nicholls, Thomas H., eds. Biology and conservation of owls of the Northern Hemisphere: 2nd International symposium. Gen. Tech. Rep. NC-190. St. Paul, MN: US Dept. of Agriculture, Forest Service, North Central Forest Experiment Station. 315-324. (Vol. 190).
- Goszczyński, J., Jabłoński, P., Lesiński, G., & Romanowski, J. (1993). Variation in diet of Tawny Owl Strix aluco L. along an urbanization gradient. Acta ornithologica, 27(2), 113-123.
- Ryszkowski, L., Wagner, C. K„ Goszczyński, J. & Truszkowski, J., (1971). Operation of predators in a forest and cultivated fields. Ann. Zool. Fennici, 8, 1: 160—168.
- Jędrzejewski, W., Jędrzejewska, B., Zub, K., Ruprecht, A. L., & Bystrowski, C. (1994). Resource use by Tawny Owls Strix aluco in relation to rodent fluctuations in Białowieża National Park, Poland. Journal of Avian Biology, 308-318.
- Wendland, V. (1972). 14jährige Beobachtungen zur Vermehrung des Waldkauzes (Strix aluco L.). Journal für Ornithologie, 113(3), 276-286.
- Gryz, J., & Krauze-Gryz, D. (2016). Wpływ pory roku i dostępności gryzoni leśnych na skład pokarmu puszczyka Strix aluco w warunkach mozaiki polno-leśnej środkowej Polski. Sylwan, 160(1), 57-63.
- Goszczynski, J. (1981). Comparative analysis of food of owls in agrocenoses. Ekol. Pol., 29(3), 431-439.
- Uttendorfer, O. (1952). Neue Ergebnisse fiber die Ernährung der Greifvögel und Eulen. Eugen Umer, Stuttgart, Germany.
- Baudvin, H., & Jouaire, S. (2006). Le régime alimentaire d'une population forestière de Chouettes hulottes (Strix aluco) en Bourgogne. Rev. Sci. Bourgogne-Nature, 4, 85-89.
- Roulin, A., Ducret, B., Bize, P., Piault, R. & Ravussin, P.A. (2008). Régime alimentaire de la chouette hulotte Strix aluco en Suisse Romande. Nos Oiseaux, 55, 149-156.
- Lundberg, A. (1980). Why are the Ural Owl Strix uralensis and the Tawny Owl S. aluco parapatric in Scandinavia? Ornis Scandinavica, 116-120.
- Sharikov, A. V., Kholopova, N. S., Volkov, S. V., & Makarova, T. V. (2009). Review of the owls' diet in the Moscow City and the Moscow Region. Volkov, SV (ed.-in-chief), Sharikov, AV & Morozov, VV (eds) Owls of The Northern Eurasia: Ecology, spatial and habitat distribution. Moscow, 188, 204.
- Scaravelli, D., & Aloise, G. (1995). Predation on dormice in Italy. Italian Journal of Mammalogy.
- Obuch, J. (2001). Dormice in the diet of owls in the Middle East. Trakya University Journal of Scientific Research Series B, 2(2), 145-150.
- Hürner, H., & Michaux, J. (2009). Ecology of the edible dormouse (Glis glis) in a western edge population in southern Belgium. Vie et Milieu, 59(2), 243-250.
- Kuhar, B., Kalan, G., & Janžekovič, F. (2006). Prehrana lesne sove Strix aluco na Kozjanskem (V Slovenija). Acrocephalus, 27(130/131), 147-154.
- Balčiauskienė, L., & Balčiauskas, L. (2008). Common dormouse as a prey item of breeding tawny owls in five districts of Lithuania. Acta Zoologica Lituanica, 18(1), 61-65.
- Juskaitis, R. (2004). Local impact of the Tawny owls (Strix aluco) on the common dormice (Muscardinus avellanarius) in Lithuania. Ekologia (Bratislava)/Ecology (Bratislava), 23(3), 305-309.
- Balčiauskas, L., Balčiauskienė, L., & Alejunas, P. (2011). Northern birch mouse (Sicista betulina) in Lithuania, findings in the diet of Tawny owl (Strix aluco). Acta Zoologica Academiae Scientiarum Hungaricae, 57(3), 277-289.
- Żmihorski, M., Gryz, J., Krauze-Gryz, D., Olczyk, A., & Osojca, G. (2011). The Tawny owl Strix aluco as a material collector in faunistic investigations: the case study of small mammals in NE Poland. Acta Zoologica Lituanica, 21(3), 185-191.
- Sarà, M., & Zanca, L. (1989). Regime alimentare dell'Allocco (Strix aluco) in Sicilia ed in Aspromonte (Calabria). Avocetta, 13, 31-39.
- Tergou, S., Boukhemza, M., Marniche, F., Milla, A., & Doumandji, S. (2014). Dietary Distinctive Features of Tawny Owl Strix aluco (Linn 1758) and Barn Owl Tyto alba (Scopoli 1759) in Gardens of Algerian Sahel El Harrach Jardin D'essai Du Hamma. Pakistan Journal of Zoology, 46(4).
- Wiącek, J., & Niedźwiedź, M. (2008). Porównanie składu pokarmu puszczyka Strix aluco w leśnej i miejskiej strefie Lublina. W: Fauna miast. Ochronić różnorodność biotyczną w miastach. P. Indykiewicz, L. Jerzak, T. Barczak (red.), SAR „Pomorze", Bydgoszcz, 501-505.
- Hirons, G. J. M. (1984). The diet of tawny owls (Strix aluco) and kestrels (Falco tinnunculus) in the New Forest, Hampshire. Proceedings of the Hampshire Field Club and Archaeological Society, 40, 21-26.
- Aksan, Ş., & Cohadar, H. (2018). New locality record of anatolian ground squirrel, Spermophilus xanthoprymnus (Bennett, 1835) in Isparta-Gencali province. Fresenius Environmental Bulletin, 27(12 A), 9172-9178.
- Hanski, I. K. (2000). Ecology of the Eurasian flying squirrel (Pteromys volans) in Finland. Biology of gliding mammals, 67-86.
- Randler, C. (2006). Anti-predator response of Eurasian red squirrels (Sciurus vulgaris) to predator calls of tawny owls (Strix aluco). Mammalian Biology, 71(5), 315-318.
- Kitowski, I., & Pitucha, G. (2007). Diet of the Eurasian Tawny Owl in farmland of east Poland. Berkut, 16(2), 225-231.
- Nedyalkov, N. (2016). Distribution and current status of Cricetulus migratorius (Mammalia: Cricetidae) in Bulgaria, with comments on its status in the Balkans. Turkish Journal of Zoology, 40(6), 925-932.
- Simeonovska-Nikolova, D., & Dekov, O. (2013). Some aspects of the behavior and defensive vocalization of the romanian hamster, Mesocricetus newtoni. Acta Zool. Bulg, 65, 462-468.
- Nedyalkov, N., & Boev, Z. (2016). Diet of Barn Owl Tyto alba and Tawny Owl Strix aluco in central Anatolia, Turkey. Crex, 1, 2-6.
- Obuch, J. (2018). On the diet of owls (Strigiformes) in Jordan. Slovak Raptor Journal, 12(1), 9-40.
- Comay, O. & Dayan, T. (2018). Owl verebrate prey taxa in the southern Levant per site. Dryad.
- Wilson, E. J., Newson, R. M., & Aliev, F. F. (1966). Enemies and competitors of the nutria in USSR. Journal of Mammalogy, 47(2), 353-355.
- Korpimäki, E., & Norrdahl, K. (1989). Avian and mammalian predators of shrews in Europe: regional differences, between-year and seasonal variation, and mortality due to predation. In Annales Zoologici Fennici (pp. 389-400). Finnish Zoological Publishing Board, formed by the Finnish Academy of Sciences, Societas Scientiarum Fennica, Societas pro Fauna et Flora Fennica and Societas Biologica Fennica Vanamo.
- Petty, S. J. (1999). Diet of tawny owls (Strix aluco) in relation to field vole (Microtus agrestis) abundance in a conifer forest in northern England. Journal of Zoology, 248(4), 451-465.
- Guerin, G. (1932). La hulotte et son régime. Ed. Elsevier Sequoïa, Paris.
- Delmee, E., Dachy, P. & Simon, P. (1979). Etude comparative du régime alimentaire de la Chouette hulotte. Rev. Gerfaut, 45 - 77.
- Queiroz, A. I., Bertrand, A., & Khakhin, G. V. (1996). Status and conservation of Desmaninae in Europe (Vol. 76). Council of Europe.
- Morris, P.A. (1997). The Hedgehog. Shire Natural History Series: Mammals 32.
- Jaksic, F. M., & Soriguer, R. C. (1981). Predation upon the European rabbit (Oryctolagus cuniculus) in Mediterranean habitats of Chile and Spain: a comparative analysis. The Journal of Animal Ecology, 269-281.
- Kowalski, K. (1995). Taphonomy of bats (Chiroptera). Geobios, 28, 251-256.
- García, A. M., Cervera, F., & Rodríguez, A. (2005). Bat predation by long-eared Owls in mediterranean and temperate regions of southern Europe. J. Rapt. Res., 39 (4): 445-453.
- Lesiński, G., Gryz, J., & Kowalski, M. (2009). Bat predation by tawny owls Strix aluco in differently human‐transformed habitats. Italian Journal of Zoology, 76(4), 415-421.
- Sharikov A.V. & Makarova T.V. (2014). Bats in the diet of owls in Northern Eurasia. Plecotus, 17: 30–36.
- Kowalski, M., & Lesinski, G. (1990). The food of the tawny owl (Strix aluco L.) from near a bat cave in Poland. Myotis, 44, 10-3.
- Spitzenberger, F., Engelberger, S., & Kugelschafter, K. (2014). Real time observations of Strix aluco preying upon a maternity colony of Myotis emarginatus. Vespertilio, 17, 185-196.
- Speakman, J.R. (1991). The impact of predation by birds on bat populations in the British Isles. Mammal Review, 21:123–142.
- Uhrin, M., Kanuch, P., Benda, P., Hapl, E., Verbeek, H. J., Kristin, A., & Andreas, M. (2006). On the Greater noctule (Nyctalus lasiopterus) in central Slovakia. Vespertilio, 9(10), 183-192.
- Korpimäki, E., & Norrdahl, K. (1989). Avian predation on mustelids in Europe 1: occurrence and effects on body size variation and life traits. Oikos, 205-215.
- Bochenski, Z. M., Tomek, T., Boev, Z., & Mitev, I. (1993). Patterns of bird bone fragmentation in pellets of the Tawny Owl (Strix aluco) and the Eagle Owl (Bubo bubo) and their taphonomic implications. Acta zoologica cracoviensia, 36(2), 313-328.
- Sasvári, L., & Hegyi, Z. (1998). Bird predation by tawny owls (Strix aluco L.) and its effect on the reproductive performance of tits. Acta Oecologica, 19(6), 483-490.
- Persson, M. (2003). Habitat quality, breeding success and density in Tawny Owl Strix aluco. Ornis Svecica, 13, 137-143.
- Schulz, V. W., & Massow, S. (1998). Nahrungsspektrum beim Waldkauz (Strix aluco) im Schloßpark Buch (Bezirk Berlin-Pankow). Berl. ornithol. Berlin, 8: 147-167.
- Zalewski, A. (1994). Diet of urban and suburban Tawny owls. Journal of Raptor Research, 28(4), 246-252.
- Grzędzicka, E., Krzysztof, K. U. S., & Nabielec, J. (2013). The effect of urbanization on the diet composition of the tawny owl (Strix aluco L.). Pol. J. Ecol, 61(2), 391-400.
- Glue, D. E. (1972). Bird prey taken by British owls. Bird Study, 19(2), 91-96.
- Comay, O., & Dayan, T. (2018). What determines prey selection in owls? Roles of prey traits, prey class, environmental variables, and taxonomic specialization. Ecology and evolution, 8(6), 3382-3392.
- Tishechkin, A. K. (1997). Comparative food niche analysis of Strix owls in Belarus. In In: Duncan, James R.; Johnson, David H.; Nicholls, Thomas H., eds. Biology and conservation of owls of the Northern Hemisphere: 2nd International symposium. Gen. Tech. Rep. NC-190. St. Paul, MN: US Dept. of Agriculture, Forest Service, North Central Forest Experiment Station. 456-460. (Vol. 190).
- Manganaro, A., Ranazzi, L., & Salvati, L. (2000). The diet of Tawny Owls (Strix aluco) breeding in different woodlands of Central Italy. Bueto, 11, 115-124.
- Kirk, D. A. (1992). Diet changes in Breeding Tawny Owls. J Raptor Res, 26(4), 239-242.
- Yalden, D. W. (1985). Dietary separation of owls in the Peak District. Bird study, 32(2), 122-131.
- Zawadzka, D., & Zawadzki, J. (2007). Feeding ecology of tawny owl (Strix aluco) in Wigry national park (North east Poland). Acta Zoologica Lituanica, 17(3), 234-241.
- Bergman, G. (1961). The food of birds of prey and owls in Fenno-Scandia. British Birds, 54, 307-320.
- Saunders, D. R. (1962). Owls feeding on young sea-birds. British Birds, 55: 591.
- Valkama, J., Korpimäki, E., Arroyo, B., Beja, P., Bretagnolle, V., Bro, E., Kenward, R., Manosa, S., Redpath, S. & Viñuela, J. (2005). Birds of prey as limiting factors of gamebird populations in Europe: a review. Biological Reviews, 80(2), 171-203.
- Alegre, J., Hernández, A., & Purroy, F. J. (1989). Datos sobre el régimen alimentario del cárabo (Strix aluco L.) en la provincia de León (NO de España). Miscel· lània Zoològica, 13, 209-211.
- Gryz, J., Góźdź, I., & Krauze-Gryz, D. (2011). Impact of anthropogenic landscape transformation on the diet composition of tawny owl Strix aluco L. in Biebrza National Park. Parki Narodowe i Rezerwaty Przyrody, 30(3/4), 109-118.
- Balčiauskienė, L., Juškaitis, R., & Atkočaitis, O. (2005). The Diet of the Tawny Owl (Strix Aluco) in South-Western Lithuania during the Breeding Period. Acta Zoologica Lituanica, 15(1), 13-20.
- Gryz, J., Lesiński, G., Kowalski, M., & Krrauze, D. (2012). Skład pokarmu puszczyka Strix aluco w Puszczy Białowieskiej. Chrońmy Przyrodę Ojczystą, 68(2), 100-108.
- Lesiński, G., Błachowski, G., & Siuchno, M. (2009). Vertebrates in the diet of the tawny owl Strix aluco in northern Podlasie (NE Poland)–comparison of forest and rural habitats. Fragm. faun, 52, 51-59.
- Solonen, T., & Karhunen, J. (2002). Effects of variable feeding conditions on the Tawny Owl Strix aluco near the northern limit of its range. Ornis Fennica, 79(3), 121-131.
- Balčiauskienė, L., & Dementavičius, D. (2006). Habitat determination of tawny owl (Strix aluco) prey composition during breeding period. Acta biologica universitatis Daugavpiliensis, 6(2), 1-12.
- Gaggi, A. & Paci, A. (2009). Note sull'orientamento trofico dell'Allocco Strix aluco in Umbria. Gli Uccelli d'Italia. XXXIV. 35-49.
- Obuch, J. (2004). Typy potravy sovy obyčajnej (Strix aluco) v Národnom parku Muránska planina. Reussia, 1 (Supplement 1), 299-309.
- Smeenk, C. (1972). Ökologische vergleiche zwischen Waldkauz Strix aluco und Waldohreule Asio otus. Ardea, 60(1-2), 1-71.
- Birrer, S. (2009). Synthesis of 312 studies on the diet of the Long-eared Owl Asio otus. Ardea, 97(4), 615-625.
- Balčiauskienė, L., Jovaišas, A., Naruševičius, V., Petraška, A., & Skuja, S. (2006). Diet of Tawny Owl (Strix aluco) and Long-eared Owl (Asio otus) in Lithuania as found from pellets. Acta zoologica lituanica, 16(1), 37-45.
- Nilsson, I. N. (1984). Prey weight, food overlap, and reproductive output of potentially competing Long-eared and Tawny Owls. Ornis Scandinavica, 176-182.
- Johnsgard, P. A. (1988). North American owls: biology and natural history. Smithsonian Institution.
- Erlinge, S., Goransson, G., Hogstedt, G., Jansson, G., Liberg, O., Loman, J., Nilsson, I.N., von Schantz, T. & Sylvén, M. (1984). Can vertebrate predators regulate their prey? The American Naturalist, 123(1), 125-133.
- Winde, H. (1970). Osteologische Untersuchungen an einigen deutschen Eulenarten. Zool. Abhandl Staal. Mus. Tierk. Dresden, 30: 149-157.
- Massemin, S., & Handrich, Y. (1997). Higher winter mortality of the Barn Owl compared to the Long-eared Owl and the Tawny Owl: Influence of lipid reserves and insulation? The Condor, 99(4), 969-971.
- Vrezec, A. L. (2003). Breeding Density and Altitudinal Distribution of Ural, Tawny and Boreal Owls in North Dinaric Alps (Central Slovenia). J. Raptor Res, 37(1), 55-62.
- Pačenovský, S. (1995). K medzidruhovým vzťahom Glaucidium passerinum, Strix uralensis a Strix aluco [To interspecific relations between Glaucidium passerinum, Strix uralensis and Strix aluco]. Tichodroma, 8, 61-73.
- Vrezec, A., & Tome, D. (2004). Habitat selection and patterns of distribution in a hierarchic forest owl guild. Ornis Fennica, 81(3), 109-118.
- Krištín, A., Mihók, J., Danko, Š., Karaska, D., Pacenovský, S., Saniga, M., Boďová, M., Balázs, C., Šotnár, K., Korňan, J. & Olekšák, M. (2007). Distribution, abundance and conservation of the Ural Owl Strix uralensis in Slovakia. Tagungsbericht des Nationalparks Bayerischer Wald, 8, 8-15.
- Penteriani, V. & Delgado, M.d.M. (2019). The Eagle-Owl. Poyser Monographs.
- Curry-Lindahl, K. Photographic Studies of Some Less Familiar Birds: LXXXIV. Eagle Owl. British Birds, L: 486-490.
- Lourenço, R., Goytre, F., del Mar Delgado, M., Thornton, M., Rabaça, J. E., & Penteriani, V. (2013). Tawny owl vocal activity is constrained by predation risk. Journal of avian biology, 44(5), 461-468.
- Sergio, F., Marchesi, L., Pedrini, P., & Penteriani, V. (2007). Coexistence of a generalist owl with its intraguild predator: distance-sensitive or habitat-mediated avoidance? Animal Behaviour, 74(6), 1607-1616.
- Mikkola, H. (1976). Owls killing and killed by other owls and raptors in Europe. British Birds, 69, 144-154.
- Lourenço, R., & Rabaça, J. (2006). Intraguild predation by eagle owls in Europe. Airo, 16, 63-68.
- Lourenço, R., Santos, S. M., Rabaça, J. E., & Penteriani, V. (2011). Superpredation patterns in four large European raptors. Population Ecology, 53(1), 175-185.
- Resano, J., Hernández-Matías, A., Real, J., & Parés, F. (2011). Using stable isotopes to determine dietary patterns in Bonelli's eagle (Aquila fasciata) nestlings. Journal of Raptor Research, 45(4), 342-353.
- Sánchez‐Zapata, J. A., Eguía, S., Blázquez, M., Moleón, M., & Botella, F. (2010). Unexpected role of ungulate carcasses in the diet of Golden Eagles Aquila chrysaetos in Mediterranean mountains. Bird Study, 57(3), 352-360.
- Chavko, J., Danko, Š., Obuch, J., & Mihók, J. (2007). The food of the Imperial Eagle (Aquila heliaca) in Slovakia. Slovak Raptor Journal, 1, 1-18.
- Sergio, F.., & Boto, A. (1999). Nest dispersion, diet, and breeding success of Black Kites (Milvus migrans) in the Italian pre-Alps. Journal of Raptor Research, 33, 207-217.
- Buker, J.B. & Hartog, A. (1985). Kauw Corvus monedula trekt in bij Bosuil Strix aluco. Limosa, 58: 74.
- Zemlyanukhin, A.I. (1995). The influence of pine marten on the number of yellow, clintukh and gray owl in the Lipetsk region. Biological Sciences, 2: 20-22.
- Jędrzejewski, W., Zalewski, A., & Jędrzejewska, B. (1993). Foraging by pine marten Martes marłeś in relation to food resources in Białowieża National Park, Poland. Acta Theriologica, 38(4), 405-426.
- Sunde, Peter (September 2005). "Predators control post-fledging mortality in tawny owls, Strix aluco". Oikos. 110 (3): 461–472. doi:10.1111/j.0030-1299.2005.14069.x.
- Baudvin, H., Dessolin, J. L., & Riols, C. (1985). L'utilisation par la martre (Martes martes) des nichoirs chouettes dans quelques forêts bourguignonnes. Ciconia, 9(2), 61-104.
- Szomjas, L. (1955). Tawny Owl attacking Marten. Aquila, 59: 450.
- Overskaug, K., Kristiansen, E., & Sunde, P. (1995). Sex-specific diet analysis of the Tawny Owl Strix aluco in Norway. Journal of Raptor Research, 29, 137-140.
- Žlender, N. (2016). Teritorialni in plenilski odzivi kozače (Strix uralensis) na manjše sintopične tekmece: diplomsko delo. univerzitetni študij (Doctoral dissertation, N. Žlender).
- Michel, V. T., Jiménez‐Franco, M. V., Naef‐Daenzer, B., & Grüebler, M. U. (2016). Intraguild predator drives forest edge avoidance of a mesopredator. Ecosphere, 7(3), e01229.
- Kitowski, I. (2002). Coexistence of owl species in the farmland of southeastern Poland. Acta ornithologica, 37(2), 121-125.
- König, C. (1998). Ecology and population of pigmy owls Glaucidium passerinum in the Black Forest (SW Germany). Holarctic Birds of Prey (Ed. by BU Meyburg, RD Chancellor & FF Ferrero), pp. 447e450. Badajoz: Adenex-WWGBP.
- Saurola, P. (1987). Mate and nest-site fidelity in Ural and Tawny owls. Biology and conservation of northern forest owls, 81-86.
- Saladin, V., Ritschard, M., Roulin, A., Bize, P., & Richner, H. (2007). Analysis of genetic parentage in the tawny owl (Strix aluco) reveals extra-pair paternity is low. Journal of Ornithology, 148(1), 113-116.
- Hirons, G. J. M. (1985). The effects of territorial behaviour on the stability and dispersion of tawny owl (Strix aluco) populations. Journal of Zoology, 1(1), 21-48.
- Camprodon, J., Salvanyà, J., & Soler-Zurita, J. (2008). The abundance and suitability of tree cavities and their impact on hole-nesting bird populations in beech forests of NE Iberian Peninsula. Acta Ornithologica, 43(1), 17-31.
- Salvati, L., Manganaro, A., & Ranazzi, L. (2002). Wood quality and the Tawny Owl Strix aluco in different forest types of central Italy. Ornis Svecica, 12, 47-51.
- Jedrzejewski, W., Jedrzejewska, B., Szymura, A., & Zub, K. (1996). Tawny owl (Strix aluco) predation in a pristine deciduous forest (Bialowieza National Park, Poland). Journal of animal ecology, 105-120.
- Mebs, T. (1980). Eulen und Käuze. Stuggart.
- Gryz, J., & Krauze-Gryz, D. (2011). Wykorzystanie skrzynek lęgowych przez puszczyki Strix aluco w środkowej Polsce. Studia i Materiały Centrum Edukacji Przyrodniczo-Leśnej, 13(2 [27]).
- Petty, S. J. (1987). The design and use of a nestbox for Tawny Owls Strix aluco in upland forests. Quarterly Journal of Forestry, 81, 103-109.
- Petty, S. J., Shaw, G., & Anderson, D. I. K. (1994). Value of nest boxes for population studies and conservation of owls in coniferous forests in Britain. Journal of Raptor Research, 28(3), 134-142.
- Sacchi, R., Galeotti, P., Boccola, S., & Baccalini, F. (2004). Occupancy rate and habitat variables influencing nest-box use by tawny owls Strix aluco. Avocetta-Parma-, 28(1), 25-30.
- Saurola>Saurola, P. (1987). Mate and nest-site fidelity in Ural and Tawny owls. Biology and conservation of northern forest owls, 81-86.
- Plesnik, J., & Dusik, M. (1994). Reproductive output of the Tawny Owl Strix aluco in relation to small mammal dynamics in intensively cultivated farmland. Meyburg B.-U. & RD Chancellor (eds.). Raptor Conservation Today. World Working Group on Birds of Prey and Owls & Pica Press, London: 531, 535.
- Grandāns, G., Keišs, O., & Avotiņš, A. (2009). Onset of breeding in Tawny Owl Strix aluco in eastern Latvia. Latvijas Universitātes Raksti, 81.
- Rockenbauch, D. (1978). Brutbiologie und den Bestand steuernde Faktoren bei Waldkauz (Strix aluco) und Waldohreule (Asio otus) in der Schwäbischen Alb. Journal für Ornithologie, 119(4), 429-440.
- Linkola, P., & Myllymäki, A. (1969). Der Einfluss der Kleinsäugerfluktuationen auf das Brüten einiger kleinsäugerfressender Vögel im südlichen Häme, Mittelfinnland 1952–1966. Ornis Fennica, 46(2), 45-78.
- Strazds, M. & Strazds, A. (1985). Birds wintering in the town of Riga. Communications of the Baltic Commission for the study of bird migration, 17: 123-136.
- Zuberogoitia, I., Martínez, J. A., Iraeta, A., Azkona, A., & Castillo, I. (2004). Possible first record of double brooding in the tawny owl Strix aluco. Ardeola, 51(2), 437-439.
- Smeenk, C. (1969). Legselgrootte bij de Bosuil (Strix aluco L.). Limosa, 42: 79-81.
- Solonen, T., & af Ursin, K. (2008). Breeding of Tawny Owls Strix aluco in rural and urban habitats in southern Finland. Bird Study, 55(2), 216-221.
- Bos, J. (1992). Over de opzienbarende kolonisatie van de Bosuil Strix aluco in Groningen en Drenthe. Drentse vogels, 5(1), 43-47.
- Solonen, T. (2005). Breeding of the Tawny Owl Strix aluco in Finland: responses of a southern colonist to the highly variable environment of the North. Ornis Fennica, 82(3), 97-106.
- Piault, R., Gasparini, J., Bize, P., Paulet, M., McGraw, K. J., & Roulin, A. (2008). Experimental support for the makeup hypothesis in nestling tawny owls (Strix aluco). Behavioral Ecology, 19(4), 703-709.
- Makatsch, W. (1976). Die Eier der Vögel Europas. Leipzig, 2.
- Southern, H. N. (1969). Prey taken by Tawny Owls during the breeding season. Ibis, 111(3), 293-299.
- de Boe, J. (1990). Recherche sur le regime alimentaire de la Chouette hulotte, Strix aluco, par la methode de a photographie sur pellicule infrarouge. In: Rapaces nocturnes. Ed. Nos oiseaux.
- van Veen, J.C. (1990). Apport de proies et croissance des jeunes chez les Chouettes hulottes, Strix aluco, reproduisant dans des etables. In Juillard, M (ed.) Rapaces nocturnes, pp.299–316. Nos Oiseaux.
- Ritter, F. (1972). Untersuchungen uber die Futterungsaktivitat des Waldkauzes (Strix aluco L.) wahrend eiener Brutperiode. Beitr. Vogelkd, 18, 156-161.
- Sasvári, L., & Hegyi, Z. (2010). Feeding effort of male Tawny Owls Strix aluco follows a fixed schedule: a field experiment in the early nestling period. Acta ornithologica, 45(2), 181-188.
- Solonen, T. (2009). Factors affecting reproduction in the tawny owl Strix aluco in southern Finland. In Annales Zoologici Fennici (Vol. 46, No. 4, pp. 302-311). Finnish Zoological and Botanical Publishing Board.
- Solonen, T. (2013). Factors affecting timing of breeding in the Tawny Owl Strix aluco. Open Ornithol J, 6, 40-51.
- Sasvári, L., Péczely, P., & Hegyi, Z. (2008). Parental testosterone and estradiol concentrations in the early nestling period correlate with the age-dependent breeding performance in Tawny Owls Strix aluco. Orn Fenn, 85, 46-54.
- Sasvári, L., Péczely, P., & Hegyi, Z. (2004). The influence of parental age and weather on testosterone concentration and offspring survival in broods of tawny owl Strix aluco. Behavioral Ecology and Sociobiology, 56(3), 306-313.
- Scherzinger, W. (1971). Beobachtungen zur Jugendentwicklung einigen Eulcn (Strigidae). Z. Tierpsychol. 28: 494-504.
- Cassidy, J. (1946). Tawny Owl nesting in factory wall. British Birds, 39: 155.
- Labitte, A (1952). Oiseaux, 22: 107–12.
- Da Silva, A., Van den Brink, V., Emaresi, G., Luzio, E., Bize, P., Dreiss, A. N., & Roulin, A. (2013). Melanin-based colour polymorphism signals aggressive personality in nest and territory defence in the tawny owl (Strix aluco). Behavioral Ecology and Sociobiology, 67(7), 1041-1052.
- Wallin, K. (1987). Defence as parental care in tawny owls (Strix aluco). Behaviour, 102(3-4), 213-230.
- Dobbs, A. (1966). Aggressive Tawny Owls. British Birds, 59: 108-109.
- Steinbach, G. (1980). Die Welt der Eulen. Verlag Hoffmann & Campe. Hamburg.
- Hosking, E. J., Newberry, C. W., & Smith, S. G. (1945). Birds of the Night. Collins.
- Boswall, J. (1965). An unusually aggressive Tawny Owl. British Birds, 58: 343-344.
- Cadman, W.A. (1934). An attacking Tawny Owl. British Birds, 28: 130-132.
- Hosking, Eric; Lane, Frank W. (1972). An Eye for a Bird: The Autobiography of a Bird Photographer. London, Hutchinson & Co. p. 20. ISBN 978-0-09-104460-2.
- Desfor, K. B., Boomsma, J. J., & Sunde, P. (2007). Tawny Owls Strix aluco with reliable food supply produce male‐biased broods. Ibis, 149(1), 98-105.
- Sasvári, L., Nishiumi, I., Péczely, P., & Hegyi, Z. (2010). Post-hatching testosterone concentration reflects nestling survival and pre-fledging offspring condition in the Tawny Owl Strix aluco. Ornis Fennica, 87(1), 26.
- Roulin, A., Bize, P., Tzaud, N., Bianchi, M., Ravussin, P. A., & Christe, P. (2005). Oxygen consumption in offspring tawny owls Strix aluco is associated with colour morph of foster mother. Journal of Ornithology, 146(4), 390-394.
- Weller, L.G. (1944). Young Tawny Owls leaving nest at early age. British Birds, 38: 80.
- Southern, H. N., Vaughan, R., & Muir, R. C. (1954). The behaviour of young Tawny Owls after fledging. Bird Study, 1(3), 101-110.
- Coles, C. F., & Petty, S. J. (1997). Dispersal behavior and survival of juvenile Tawny owls (Strix aluco) during the low point in a vole cycle. In In: Duncan, James R.; Johnson, David H.; Nicholls, Thomas H., eds. Biology and conservation of owls of the Northern Hemisphere: 2nd International symposium. Gen. Tech. Rep. NC-190. St. Paul, MN: US Dept. of Agriculture, Forest Service, North Central Forest Experiment Station. 111-118. (Vol. 190).
- Overskaug, K., Bolstad, J. P., Sunde, P., & ⵁien, I. J. (1999). Fledgling behavior and survival in northern tawny owls. The Condor, 101(1), 169-174.
- Petty, S. J., & Thirgood, S. J. (1989). A radio tracking study of post‐fledging mortality and movements of Tawny Owls in Argyll. Ringing & Migration, 10(2), 75-82.
- Sunde, P., & Naundrup, P. J. (2016). Spatial and begging behaviours of juvenile Tawny Owls (Strix aluco) from fledging to independence under contrasting food conditions. Journal of Ornithology, 157(4), 961-970.
- Sunde, P. (2008). Parent‐offspring conflict over duration of parental care and its consequences in tawny owls Strix aluco. Journal of Avian Biology, 39(2), 242-246.
- Sunde, P., & Markussen, B. E. (2005). Using counts of begging young to estimate post-fledging survival in tawny owls Strix aluco. Bird Study, 52(3), 343-345.
- Sunde, P. (1999). Overlevelse og spredning af radiomærkede unge Natugler Strix aluco i Nordsjælland. Danish with an English summary: Survival and dispersal of young tawny owls-preliminary results. Dansk Omitologisk Tidsskrift, 93, 267-270.
- Coles, C. F., Petty, S. J., MacKinnon, J. L., & Thomas, C. J. (2003). The role of food supply in the dispersal behaviour of juvenile Tawny Owls Strix aluco. Ibis, 145(2), E59-E68.
- Sasvári, L., Hegyi, Z., Csörgõ, T., & Hahn, I. (2000). Age-dependent diet change, parental care and reproductive cost in tawny owls Strix aluco. Acta Oecologica, 21(4-5), 267-275.
- Sasvari, L., & Hegyi, Z. (2002). Effects of age composition of pairs and weather condition on the breeding performance of tawny owls, Strix aluco. Folia Zoologica-Praha-, 51(2), 113-120.
- Sasvári, L., & Hegyi, Z. (2010). Parents raise higher proportion of high quality recruits from low fledgling production in the local population of tawny owls, Strix aluco. Folia Zoologica, 59(3), 206-215.
- Grašytė, G., Rumbutis, S., Dagys, M., & Treinys, R. (2016). Breeding performance, apparent survival, nesting location and diet in a local population of the Tawny Owl Strix aluco in Central Lithuania over the long-term. Acta Ornithologica, 51(2), 163-175.
- Baudvin, H. (1990). L’influence du regime alimentaire sur la reproduction des Chouettes hulottes, Strix aluco dans les foreˆts bourguignonnes. In Juillard, M. (ed.) Rapaces nocturnes, pp. 33–6. Ed. Nos Oiseaux. Scriptura, S.A., Porrentruy (JU), Switzerland.
- Roulin, A., Ducret, B., Ravussin, P. A., & Altwegg, R. (2003). Female colour polymorphism covaries with reproductive strategies in the tawny owl Strix aluco. Journal of Avian Biology, 34(4), 393-401.
- Fanfani, A., Manes, F., Moretti, V., Ranazzi, L., & Salvati, L. (2015). Vegetation, precipitation and demographic response of a woodland predator: Tawny Owl Strix aluco as an indicator of soil aridity in Castelporziano forest. Rendiconti Lincei, 26(3), 391-397.
- Ranazzi, L., Manganaro, A. & Salvati, L. (2000). The breeding success of tawny owls (Strix aluco) in a Mediterranean area: a long-term study in urban Rome. J Raptor Res, 34(4), 322-326.
- Baudvin, H., & Jouaire, S. (2003). Breeding biology of the Tawny Owl Strix aluco in the forests of Burgundy. Vogelwelt, 124, 289-294.
- Wendland, V. (1980). Der Waldkauz (Strix aluco) im bebauten Stadtgebiet von Berlin (West). Beiträge zur Vogelkunde, 26(3/4), 157-171.
- Saurola, P., & Francis, C. (2018). Towards integrated population monitoring based on the fieldwork of volunteer ringers: productivity, survival and population change of Tawny Owls Strix aluco and Ural Owls Strix uralensis in Finland. Bird Study, 65(sup1), S63-S76.
- Petty, S. J. & Peace, A.J. (1989). Productivity and density of tawny owls Strix aluco in relation to the structure of a spruce forest in Britain. In Annales Zoologici Fennici (pp. 227-233). Finnish Zoological Publishing Board, formed by the Finnish Academy of Sciences, Societas Scientiarum Fennica, Societas pro Fauna et Flora Fennica and Societas Biologica Fennica Vanamo.
- Appleby, B. M., Petty, S. J., Blakey, J. K., Rainey, P., & MacDonald, D. W. (1997). Does variation of sex ratio enhance reproductive success of offspring in tawny owls (Strix aluco). Proceedings of the Royal Society of London. Series B: Biological Sciences, 264(1385), 1111-1116.
- Petty, S. J., & Thomas, C. J. (2003). Distribution, numbers and breeding performance of Tawny Owls (Strix aluco) in relation to clear-cutting in a conifer forest in northern England. Birds of Prey in a Changing Environment’.(Eds DBA Thompson, SM Redpath, AH Fielding, M. Marquiss and CA Galbraith.) pp, 111-129.
- Jeanmonod, J. (1993). Nouvelles donnees suisses sur la longevite de la Chouette hulotte (Strix aluco) dans la nature. Nos Oiseaux, 42: 121-123.
- Lahti, E. & Mikkola, H. (1974). Viirulehtoja lapinpöllön pesäpaikoista ja pesimisbiotoopeista (Summary : Nest sites and nesting habitats of the Ural, Tawny and Great Grey Owls). Savon luonto 6: 1-10.
- Edberg, R. (1958). Om dodligheten under vinterhalvaret hos kattigglot (Strix a. aluco L.) i Mellansverige. Var Fagelv., 17: 273-280.
- GaramszegI, László Z. (2011). "Climate change increases the risk of malaria in birds". Global Change Biology. 17 (5): 1751–1759. Bibcode:2011GCBio..17.1751G. doi:10.1111/j.1365-2486.2010.02346.x.
- Francis, C. M., & Saurola, P. (2004). Estimating components of variance in demographic parameters of Tawny Owls, Strix aluco. Animal Biodiversity and Conservation, 27(1), 489-502.
- Petty, S. J., Appleby, B. M., Coles, C. F., & Julliard, R. (2004). The long-term effect of fitting back-mounted radio tags to juvenile tawny owls Strix aluco. Wildlife biology, 10(1), 161-170.
- Ewald, J. A., & Crompton, D. W. T. (1993). Centrorhynchus aluconis (Acanthocephala) and other helminth species in tawny owls (Strix aluco) in Great Britain. The Journal of parasitology, 952-954.
- Couper, D., & Gibbons, L. M. (2010). Tetrameres species parasites in tawny owls (Strix aluco).
- Aubert, D., Terrier, M. E., Dumètre, A., Barrat, J., & Villena, I. (2008). Prevalence of Toxoplasma gondii in raptors from France. Journal of wildlife diseases, 44(1), 172-173.
- Baylis, H. A. (1939). XXXVIII.—Further records of parasitic worms from British vertebrates. Journal of Natural History, 4(23), 473-498.
- Karadjian, G., Puech, M. P., Duval, L., Chavatte, J. M., Snounou, G., & Landau, I. (2013). Haemoproteus syrnii in Strix aluco from France: morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite, 20.
- Yildirim, A., Aysul, N., Bayramli, G., Inci, A., Eren, H., Aypak, S., Duzlu, O., Ciloglu, A. & Onder, Z. (2013). Detection and molecular characterization of a Haemoproteus lineage in a Tawny Owl (Strix aluco) in Turkey. Vet J Ankara Univ, 69, 179-183.
- Hurnikova, Z., Hrčková, G., Ågren, E., Komorová, P., Forsman, J., Chovancová, B., & Letková, V. (2014). First finding of Trichinella pseudospiralis in two Tawny Owls (Strix aluco) from Sweden. Helminthologia, 51(3), 190-197.
- Kolářová, I. (1982). First record of Eimeria strigis Kutzer, 1963 from tawny owl Strix aluco L. in Czechoslovakia. Folia Parasitologica, 29(4).
- Appleby, B. M., Anwar, M. A., & Petty, S. J. (1999). Short‐term and long‐term effects of food supply on parasite burdens in Tawny Owls, Strix aluco. Functional Ecology, 13(3), 315-321.
- McInnes, F. J., Crompton, D. W. T., & Ewald, J. A. (1994). The Distribution of Centrorhynchus aluconis (Acanthocephala) and Porrocaecum spirale (Nematoda) in Tawny Owls (Strix aluco) from Great Britain. Journal of Raptor Research, 28(1), 34-38.
- Tadros, W., & Laarman, J. J. (1980, January). The Tawny Owl, Strix aluco, as final host of a new species of Sarcocystis with Mus musculus as intermediate. In Tropical and Geographical Medicine (Vol. 32, No. 4, pp. 364-364). C/O Royal Tropical Inst, Kit Press, Mauritskade 63, 1092 Ad Amsterdam, Netherlands: Tropical Geographical Medicine.
- Cousquer, G. O., Cooper, J. E., & Cobb, M. A. (2010). Conjunctival flora in tawny owls (Strix aluco).
- Grzywaczewski, G., Kowalczyk-Pecka, D., Cios, S., Bojar, W., Jankuszew, A., Bojar, H., & Kolejko, M. (2017). Tawny owl Strix aluco as a potential transmitter of Enterobacteriaceae epidemiologically relevant for forest service workers, nature protection service and ornithologists. Annals of Agricultural and Environmental Medicine, 24(1), 62-65.
- Petty, S. J., & Saurola, P. (1997). Strix aluco: Tawny Owl. The EBCC atlas of European breeding birds: their distribution and abundance. London: Poyser.
- "RSPB Red Amber & Green List". RSPB Red Amber & Green Lists Explained. Royal Society for the Protection of Birds. Retrieved 10 December 2017.
- Freeman, S. N., Balmer, D. E., & Crick, H. Q. (2006). On the censusing of Tawny Owl Strix aluco. Bird Census News, 19(2), 58-62.
- Worthington-Hill, J., & Conway, G. (2017). Tawny Owl Strix aluco response to call-broadcasting and implications for survey design. Bird Study, 64(2), 205-210.
- Percival, S. M. (1990). Population Trends in British Barn Owls, Tyto Alba, and Tawny Owls Strix Aluco, in Relation to Environmental Change. British Trust for Ornithology.
- Fuller, R. J., Noble, D. G., Smith, K. W., & Vanhinsbergh, D. (2005). Recent declines in populations of woodland birds in Britain. British Birds, 98, 116-143.
- Jensen, R. A., Sunde, P., & Nachman, G. (2012). Predicting the distribution of Tawny Owl (Strix aluco) at the scale of individual territories in Denmark. Journal of ornithology, 153(3), 677-689.
- Glue, D. (1973). Seasonal mortality in four small birds of prey. Ornis Scandinavica, 97-102.
- McGhie, H. (1999). Persecution of birds of prey in north Scotland 1912-69 as evidenced by taxidermists' stuffing books. Scottish birds, 20(2), 98-110.
- Glue, D. E. (1971). Ringing recovery circumstances of small birds of prey. Bird Study, 18(3), 137-146.
- Marzluff, J. M. (2001). Worldwide urbanization and its effects on birds. In Avian ecology and conservation in an urbanizing world (pp. 19-47). Springer, Boston, MA.
- Leighton, K., Chilvers, D., Charles, A., & Kelly, A. (2008). Post-release survival of hand-reared tawny owls (Strix aluco) based on radio-tracking and leg-band return data. Animal welfare, 17(3), 207-214.
- Griffiths, R., Murn, C., & Clubb, R. (2010). Survivorship of rehabilitated juvenile Tawny Owls (Strix aluco) released without support food, a radio tracking study. Avian Biology Research, 3(1), 1-6.
- Illner, H. (1992). Road deaths of Westphalian owls: methodological problems, influence of road type and possible effects of population levels. Pages 94-100 in C.A. Galbraith, I. Taylor, and S. Percival [EDS.], The ecology and conservation of European owls. Joint Nature Conservation Committee, Peterborough, U.K.
- Williams, D. L., Villavincencio, C. G., & Wilson, S. (2006). Chronic ocular lesions in tawny owls (Strix aluco) injured by road traffic. Veterinary Record, 159(5), 148-153.
- Cousquer, G. (2005). Ophthalmological findings in free-living tawny owls (Strix aluco) examined at a wildlife veterinary hospital. Veterinary record, 156(23), 734-739.
- Baudvin, H., & Jouaire, S. (2003). Les causes de mortalité chez les chouettes hulottes adultes Strix aluco dans quelques forêts de Bourgogne. Alauda, 71(2), 221-226.
- Santos, S. M., Lourenço, R., Mira, A., & Beja, P. (2013). Relative effects of road risk, habitat suitability, and connectivity on wildlife roadkills: the case of tawny owls (Strix aluco). PLoS One, 8(11), e79967.
- Silva, C. C., Lourenço, R., Godinho, S., Gomes, E., Sabino-Marques, H., Medinas, D., Neves, M., Silva, C., Rabaca, J.E. & Mira, A. (2012). Major roads have a negative impact on the Tawny Owl Strix aluco and the Little Owl Athene noctua populations. Acta ornithologica, 47(1), 47-54.
- Fröhlich, A., & Ciach, M. (2018). Noise pollution and decreased size of wooded areas reduces the probability of occurrence of Tawny Owl Strix aluco. Ibis, 160(3), 634-646.
- Conrad, B. (1977) Die Giftbelastung der Vogelwelt Deutschlands. Vogelkundliche Bibliothek Vol. 5, Kilda-Verlag, Greven.
- Debén, S., Fernández, J. Á., Aboal, J. R., & Carballeira, A. (2012). Evaluation of different contour feather types for biomonitoring lead exposure in Northern goshawk (Accipiter gentilis) and tawny owl (Strix aluco). Ecotoxicology and environmental safety, 85, 115-119.
- Varela, Z., García-Seoane, R., Fernández, J. A., Carballeira, A., & Aboal, J. R. (2016). Study of temporal trends in mercury concentrations in the primary flight feathers of Strix aluco. Ecotoxicology and environmental safety, 130, 199-206.
- Seoane, R. G., Río, Z. V., Ocaña, A. C., Escribano, J. Á. F., & Viñas, J. R. A. (2018). Selection of tawny owl (Strix aluco) flight feather shaft for biomonitoring As, Cd and Pb pollution. Environmental Science and Pollution Research, 25(14), 14271-14276.
- Ahrens, L., Herzke, D., Huber, S., Bustnes, J. O., Bangjord, G., & Ebinghaus, R. (2011). Temporal trends and pattern of polyfluoroalkyl compounds in tawny owl (Strix aluco) eggs from Norway, 1986− 2009. Environmental science & technology, 45(19), 8090-8097.
- Walker, L. A., Turk, A., Long, S. M., Wienburg, C. L., Best, J., & Shore, R. F. (2008). Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Science of the Total Environment, 392(1), 93-98.
- Eriksson, U., Roos, A., Lind, Y., Hope, K., Ekblad, A., & Kärrman, A. (2016). Comparison of PFASs contamination in the freshwater and terrestrial environments by analysis of eggs from osprey (Pandion haliaetus), tawny owl (Strix aluco), and common kestrel (Falco tinnunculus). Environmental research, 149, 40-47.
- Hahn, E., Hahn, K., & Stoeppler, M. (1993). Bird feathers as bioindicators in areas of the German environmental specimen bank-bioaccumulation of mercury in food chains and exogenous deposition of atmospheric pollution with lead and cadmium. Science of the Total Environment, 139, 259-270.
- Armstrong, Edward A. (1958). The Folklore of Birds: An Enquiry into the Origin and Distribution of Some Magico-Religious Traditions. London: Collins. p. 114.
- Wordsworth, William; Coleridge, Samuel Taylor (1800). Lyrical Ballads. London: Longman.
External links
| Wikimedia Commons has media related to Strix aluco. |
| Wikispecies has information related to Strix aluco. |
| Look up tawny owl in Wiktionary, the free dictionary. |
