Triptolide
Triptolide is a diterpenoid epoxide which is produced by the thunder god vine, Tripterygium wilfordii. It has in vitro and in vivo activities against mouse models of polycystic kidney disease[2] and pancreatic cancer, but its physical properties[3] and severe toxicity[4] limit its therapeutic potential.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.208.723 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H24O6 | |
| Molar mass | 360.406 g·mol−1 |
| 0.017 mg/mL[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Consequently, a synthetic water-soluble prodrug, minnelide, is being studied clinically instead.[3][5]
Mechanism of action
Several putative target proteins of triptolide have been reported, including polycystin-2,[6] ADAM10,[7] DCTPP1,[8] TAB1,[9] and XPB.[10][11] Multiple triptolide-resistant mutations exist in XPB (ERCC3) and its partner protein GTF2H4.[12] However, no triptolide-resistant mutations were found in polycystin-2, ADAM10, DCTPP1 and TAB1. Cys342 of XPB was identified as the residue that undergoes covalent modification by the 12,13-epoxide group of triptolide, and the XPB-C342T mutant rendered the T7115 cell line nearly completely resistant to triptolide.[10] The level of resistance conferred by the C342T mutation is about 100-fold higher than the most triptolide-resistant mutants previously identified.[12] Together, these results validate XPB as a target responsible for the antiproliferative activity of triptolide. The disruption of super-enhancer networks has also been suggested as a mechanism of action.[13]
Water-soluble prodrugs
Minnelide is a more water-soluble synthetic prodrug of triptolide which is converted to triptolide in vivo.[3][14] In a preclinical mouse model of pancreatic cancer, it was "even more effective than gemcitabine". Its Phase II clinical trials are expected to conclude in February 2019.[15]
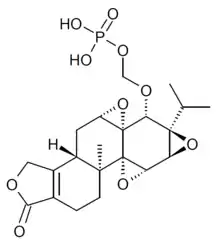
Glutriptolide, a glucose conjugate of triptolide with better solubility and lower toxicity, did not inhibit XPB activity in vitro, but exhibited tumor control in vivo, which is likely due to sustained stepwise release of active triptolide within cancer cells.[16] A second generation glutriptolide has been recently reported for targeting hypoxic cancer cells with increased glucose transporter expression.[17]
References
- Patil, Satish; Lis, Lev G.; Schumacher, Robert J.; Norris, Beverly J.; Morgan, Monique L.; Cuellar, Rebecca A. D.; Blazar, Bruce R.; Suryanarayanan, Raj; Gurvich, Vadim J.; Georg, Gunda I. (10 December 2015). "Phosphonooxymethyl Prodrug of Triptolide: Synthesis, Physicochemical Characterization, and Efficacy in Human Colon Adenocarcinoma and Ovarian Cancer Xenografts". Journal of Medicinal Chemistry. 58 (23): 9334–9344. doi:10.1021/acs.jmedchem.5b01329. PMC 4678411. PMID 26596892.
- Leuenroth, Stephanie (2007). "Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease". PNAS. 104 (11): 4389–4394. doi:10.1073/pnas.0700499104. PMC 1838612. PMID 17360534. Retrieved 18 October 2012.
- Chugh, Rohit (2012). "A Preclinical Evaluation of Minnelide as a Therapeutic Agent Against Pancreatic Cancer". Science Translational Medicine. 4 (156): 156ra139. doi:10.1126/scitranslmed.3004334. PMC 3656604. PMID 23076356. Retrieved 18 October 2012.
- Liu Q. (2011). "Triptolide and its expanding multiple pharmacological functions". International Immunopharmacology. 11 (3): 377–383. doi:10.1016/j.intimp.2011.01.012. PMID 21255694.
- "Study of Minnelide in Patients With Advanced GI Tumors". Retrieved 6 October 2016.
- S. J. Leuenroth, D. Okuhara, J. D. Shotwell, G. S. Markowitz, Z. Yu, S. Somlo, C. M. Crews, Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A 2007, 104, 4389-4394;
- R. Soundararajan, R. Sayat, G. S. Robertson, P. A. Marignani,Triptolide: An inhibitor of a disintegrin and metalloproteinase 10 (ADAM10) in cancer cells. Cancer Biol Ther 2009, 8, 2054-2062;
- T. W. Corson, H. Cavga, N. Aberle, C. M. Crews, Triptolide directly inhibits dCTP pyrophosphatase. ChemBioChem 2011, 12, 1767-1773;
- Y. Lu, Y. Zhang, L. Li, X. Feng, S. Ding, W.Zheng, J. Li, P. Shen,TAB1: A Target of Triptolide in Macrophages. Chem. Biol. 2014, 21, 246 – 256.
- Q. L. He, D. V. Titov, J. Li, M. Tan, Z. Ye, Y. Zhao, D. Romo, and J. O. Liu. Covalent Modification of a Cysteine Residue in the XPB Subunit of the General Transcription Factor TFIIH Through Single Epoxide Cleavage of the Transcription Inhibitor Triptolide. Angew. Chem. Int. Ed. 2015, 54, 1859 –1863
- D. V. Titov, B. Gilman, Q. L.He, S. Bhat,W. K. Low, Y. Dang,M.Smeaton, A. L. Demain, P. S. Miller, J. F. Kugel, J. A. Goodrich,J. O. Liu, XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 2011, 7, 182 – 188.
- Y. Smurnyy, M. Cai, H. Wu, E. McWhinnie, J. A. Tallarico, Y.Yang, Y. Feng, DNA sequencing and CRISPR-Cas9 gene editing for target validation in mammalian cells. Nat. Chem. Biol. 2014, 10, 623 – 625
- Noel, Pawan; Hussein, Shaimaa; Ng, Serina; Antal, Corina E.; Lin, Wei; Rodela, Emily; Delgado, Priscilla; Naveed, Sanna; Downes, Michael; Lin, Yin; Evans, Ronald M.; Von Hoff, Daniel D.; Han, Haiyong (9 November 2020). "Triptolide targets super-enhancer networks in pancreatic cancer cells and cancer-associated fibroblasts". Oncogenesis. 9 (11): 1–12. doi:10.1038/s41389-020-00285-9.
- Thunder God Vine Drug Zaps Pancreatic Cancer. GenEng. 2012
- "A Phase II, International Open Label Trial of Minnelide in Patients With Refractory Pancreatic Cancer". ClinicalTrials.gov. Retrieved 13 March 2018.
- He, Qing-Li; Minn, Il; Wang, Qiaoling; Xu, Peng; Head, Sarah A; Datan, Emmanuel; Yu, Biao; Pomper, Martin G; Liu, Jun O (2016). "Targeted Delivery and Sustained Antitumor Activity of Triptolide through Glucose Conjugation". Angewandte Chemie. 128 (39): 12214. doi:10.1002/ange.201606121.
- Datan E, Minn I, Peng X, He QL, Ahn H, Yu B, Pomper MG, Liu JO (2020). "A Glucose-Triptolide Conjugate Selectively Targets Cancer Cells under Hypoxia". iScience. 23 (9). doi:10.1016/j.isci.2020.101536. PMID 33083765.