Cannabichromene
Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene,[1] is one of the hundreds of cannabinoids found in the Cannabis plant,[2] and is therefore a phytocannabinoid. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others.[2][3] CBC and its derivatives are as abundant as cannabinols in cannabis.[2] It is not scheduled by the Convention on Psychotropic Substances.
-Cannabichromene.svg.png.webp) | |
| Names | |
|---|---|
| IUPAC name
2-Methyl-2-(4-methylpent-3-enyl)-7-pentyl-5-chromenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.236.929 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H30O2 | |
| Molar mass | 314.469 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
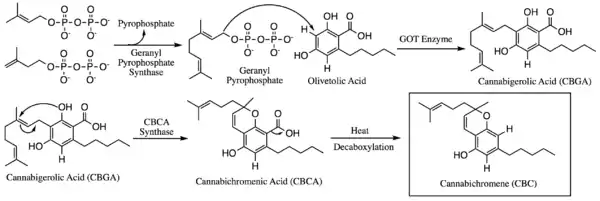
Within the Cannabis plant, CBC occurs mainly as cannabichromenic acid (CBCA, 2-COOH-CBC, CBC-COOH). Geranyl pyrophosphate and olivetolic acid combine to produce cannabigerolic acid (CBGA; the sole intermediate for all other phytocannabinoids), which is cyclized by the enzyme CBCA synthase to form CBCA. Over time, or when heated above 200° F, CBCA is decarboxylated, producing CBC. See also the biosynthetic scheme image below.
Pharmacology
As of 2017, CBC is under laboratory research to identify its possible pharmacological properties. No in vivo human studies exist as of 2019.[1][2][4][5] In vitro, CBC is not active at CB1 or CB2 receptors, but is an agonist of TRPA1 and less potently, an agonist of TRPV3 and TRPV4.[2] CBC has two stereoisomers.
References
- "Cannabichromene". PubChem. National Center for Biotechnology Information. 16 February 2019. Retrieved 12 February 2019.
- Turner, Sarah E.; Williams, Claire M.; Iversen, Leslie; Whalley, Benjamin J. (2017). "Molecular Pharmacology of Phytocannabinoids". In Kinghorn, A. Douglas; Falk, Heinz; Gibbons, Simon; Kobayashi, Jun'ichi (eds.). Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Progress in the Chemistry of Organic Natural Products. 103. Springer International Publishing. pp. 61–101. doi:10.1007/978-3-319-45541-9_3. ISBN 978-3-319-45539-6. PMID 28120231.
- Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- Morales, Paula; Hurst, Dow P.; Reggio, Patricia H. (2017). "Molecular Targets of the Phytocannabinoids: A Complex Picture". In Kinghorn, A. Douglas; Falk, Heinz; Gibbons, Simon; Kobayashi, Jun'ichi (eds.). Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Progress in the Chemistry of Organic Natural Products. 103. Springer International Publishing. pp. 103–131. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6. PMC 5345356. PMID 28120232.
- Delong, G. T.; Wolf, C. E.; Poklis, A.; Lichtman, A. H. (2010). "Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)-tetrahydrocannabinol". Drug and Alcohol Dependence. 112 (1–2): 126–33. doi:10.1016/j.drugalcdep.2010.05.019. PMC 2967639. PMID 20619971.