2-Methyl-1-butanol
2-Methyl-1-butanol (IUPAC name, also called active amyl alcohol) is an organic chemical compound.
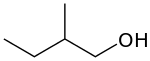 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbutan-1-ol | |
| Other names
2-Methyl-1-butanol Active amyl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.809 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.148 g/mol |
| Appearance | colorless liquid |
| Density | 0.8152 g/cm3 |
| Melting point | −117.2 °C (−179.0 °F; 156.0 K) |
| Boiling point | 127.5 °C (261.5 °F; 400.6 K) |
| 31 g/L | |
| Solubility | miscible with ethanol, diethyl ether; very soluble in acetone |
| Vapor pressure | 3 mm Hg |
| Viscosity | 4.453 mPa·s |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-356.6 kJ·mol−1 (liquid) -301.4 kJ·mol−1 (gas) |
| Hazards | |
| 385 °C (725 °F; 658 K) | |
| Related compounds | |
Related compounds |
Amyl alcohol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is one of the components of the aroma of Tuber melanosporum, the black truffle.
Uses
It is used as a solvent and an intermediate in the manufacture of other chemicals. 2-Methyl-1-butanol is a component of many mixtures of amyl alcohols sold industrially.
Reactions
2-Methyl-1-butanol can be derived from fusel oil (because it occurs naturally in fruits such as grapes[3]) or manufactured by either the oxo process or via the halogenation of pentane.[2]
See also
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3–374, 5–42, 6–188, 8–102, 16–22, ISBN 0-8493-0594-2
- McKetta, John J.; Cunningham, William Aaron (1977), Encyclopedia of Chemical Processing and Design, 3, Boca Raton, Florida: CRC Press, pp. 279–280, ISBN 978-0-8247-2480-1, retrieved 2009-12-14
- Howard, Philip H. (1993), Handbook of Environmental Fate and Exposure Data for Organic Chemicals, 4, Boca Raton, Florida: CRC Press, pp. 392–396, ISBN 978-0-87371-413-6, retrieved 2009-12-14
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
