Amyl alcohol
An amyl alcohol is any of 8 alcohols with the formula C5H12O.[1] A mixture of amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetate and other important products. The name amyl alcohol without further specification applies to the normal (straight-chain) form, 1-pentanol.
These are the 8 alcohols that are structural isomers with molecular formula C5H12O:
Amyl alcohol isomers Common name Structure Type IUPAC name Boiling point (°C)[2] 1-pentanol
or normal amyl alcohol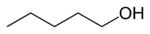
primary Pentan-1-ol 138.5 2-methyl-1-butanol
or active amyl alcohol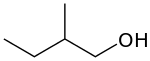
primary 2-Methylbutan-1-ol 128.7 3-methyl-1-butanol
or isoamyl alcohol
or isopentyl alcohol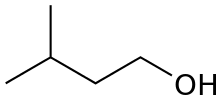
primary 3-Methylbutan-1-ol 131.2 2,2-dimethyl-1-propanol
or neopentyl alcohol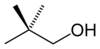
primary 2,2-Dimethylpropan-1-ol 113.1 2-pentanol
or sec-amyl alcohol
or or methyl (n) propyl carbinol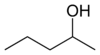
secondary Pentan-2-ol 118.8 3-methyl-2-butanol
or sec-isoamyl alcohol
or methyl isopropyl carbinol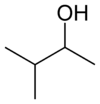
secondary 3-Methylbutan-2-ol 113.6 3-Pentanol 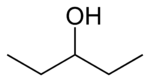
secondary Pentan-3-ol 115.3 2-methyl-2-butanol
or tert-amyl alcohol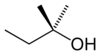
tertiary 2-Methylbutan-2-ol 102
Three of these alcohols, 2-methyl-1-butanol, 2-pentanol, and 3-methyl-2-butanol (methyl isopropyl carbinol), contain an asymmetric carbon atom and are therefore optically active.
In addition to those, the structural isomers of C5H
12O include the ethers methyl butyl ether, methyl sec-butyl ether, ethyl propyl ether, ethyl isopropyl ether, and methyl tert-butyl ether.
The most important amyl alcohol is isoamyl alcohol, the chief one generated by fermentation in the production of alcoholic beverages and a constituent of fusel oil. The other amyl alcohols may be obtained synthetically. Of these, tertiary butyl carbinol has been the most difficult to obtain, with the first reported synthesis in 1891 by L. Tissier[3] using the reduction of a mixture of trimethyl acetic acid and trimethylacetyl chloride with sodium amalgam. It is a solid that melts at 48 to 50 °C and boils at 112.3 °C.[4]
Notes
- Merriam-Webster's Collegiate Dictionary 11th Ed. 2004
- Calculated boiling points from ChemSpider.
- Comptes Rendus, 1891, 112, p. 1065
-
 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Amyl Alcohols". Encyclopædia Britannica. 1 (11th ed.). Cambridge University Press. p. 900.
One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Amyl Alcohols". Encyclopædia Britannica. 1 (11th ed.). Cambridge University Press. p. 900.
