CBD-DMH
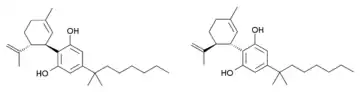
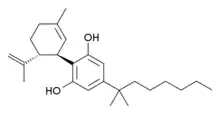
Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory.[1][2][3][4][5] Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies.[6]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H38O2 |
| Molar mass | 370.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
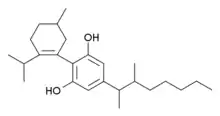
Another closely analogous compound has also been described, with the double bond in the cyclohexene ring shifted to between the 1,6-positions rather than the 2,3-positions (i.e. analogous to synthetic THC analogues such as parahexyl), the isopropenyl group saturated to isopropyl, and a 1,2-dimethylheptyl side chain. It is synthesized by Birch reduction from the 1,2-dimethylheptyl analogue of cannabidiol. This compound also produces potent cannabinoid-like effects in animals, but has three chiral centers and is composed of a mixture of eight stereoisomers, which have not been studied individually, so it is not known which stereoisomers are active.[7][8]
See also
References
- Leite, JR; Carlini, EA; Lander, N; Mechoulam, R (1982). "Anticonvulsant effects of the (−) and (+)isomers of cannabidiol and their dimethylheptyl homologs". Pharmacology. 24 (3): 141–6. doi:10.1159/000137588. PMID 7071126.
- Bisogno, T; Hanus, L; De Petrocellis, L; Tchilibon, S; Ponde, DE; Brandi, I; Moriello, AS; Davis, JB; Mechoulam, R; Di Marzo, V (2001). "Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide". Br J Pharmacol. 134 (4): 845–52. doi:10.1038/sj.bjp.0704327. PMC 1573017. PMID 11606325.
- Fride, E; Ponde, D; Breuer, A; Hanus, L (2005). "Peripheral, but not central effects of cannabidiol derivatives: mediation by CB(1) and unidentified receptors". Neuropharmacology. 48 (8): 1117–29. doi:10.1016/j.neuropharm.2005.01.023. PMID 15910887. S2CID 16531395.
- Ben-Shabat, S; Hanus, LO; Katzavian, G; Gallily, R (2006). "New cannabidiol derivatives: synthesis, binding to cannabinoid receptor, and evaluation of their antiinflammatory activity". J Med Chem. 49 (3): 1113–7. doi:10.1021/jm050709m. PMID 16451075.
- Juknat, A; Kozela, E; Kaushansky, N; Mechoulam, R; Vogel, Z (2016). "Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol - studies in BV-2 microglia and encephalitogenic T cells". J Basic Clin Physiol Pharmacol. 27 (3): 289–96. doi:10.1515/jbcpp-2015-0071. PMID 26540221.
- Hanus, LO; Tchilibon, S; Ponde, DE; Breuer, A; Fride, E; Mechoulam, R (Feb 2005). "Enantiomeric Cannabidiol Derivatives: Synthesis and Binding to Cannabinoid Receptors". Org Biomol Chem. 3 (6): 1116–1123. doi:10.1039/B416943C. PMID 15750656.
- Razdan RK, Pars HG, Thompson WR, Granchelli FE (1974). "Lithium-ammonia reduction of tetrahydrocannabinols". Tetrahedron Letters. 15 (49–50): 4315–4318. doi:10.1016/S0040-4039(01)92152-5.
- Razdan, K. (1981). "The Total Synthesis of Cannabinoids". In John Apsimon (ed.). The Total Synthesis of Natural Products. Wiley Interscience. p. 245. ISBN 978-0-471-05460-3. OCLC 19487018.