APP-FUBINACA
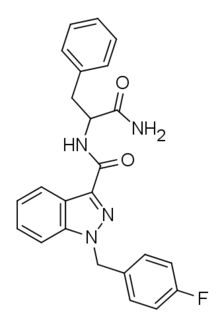
APP-FUBINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug.[1] Pharmacological testing showed APP-FUBINACA to have only moderate affinity for the CB1 receptor, with a Ki of 708 nM, while its EC50 was not tested.[2] It contains a phenylalanine amino acid residue in its structure.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H21FN4O2 |
| Molar mass | 416.456 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Legality
Sweden's public health agency suggested to classify APP-FUBINACA as hazardous substance on March 24, 2015.[3]
See also
References
- "APP-FUBINACA". Cayman Chemical. Retrieved 15 July 2015.
- WO 2009106982, Buchler IP, Hayes MJ, Hedge SG, Hockerman SL, Jones DL, Kortum SW, Rico JG, Tenbrink RE, Wu KK, "Indazole derivatives", published 3 September 2009, assigned to Pfizer Inc
- "Fler ämnen föreslås bli klassade som narkotika eller hälsofarlig vara". Folkhälsomyndigheten. Retrieved 16 July 2015.
- Cannaert A, Sparkes E, Pike E, Luo JL, Fang A, Kevin RC, et al. (December 2020). "in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA". ACS Chemical Neuroscience. 11 (24): 4434–4446. doi:10.1021/acschemneuro.0c00644. PMID 33253529.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.