Cannabivarin
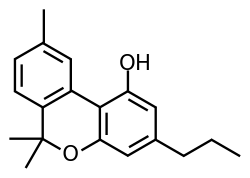
Cannabivarin (CBV), also known as cannabivarol, is considered a non-psychoactive cannabinoid — it does not produce the euphoric side effects found in THC. Minor amounts of CBV are found in the hemp plant Cannabis sativa. It is an analog of cannabinol (CBN) with the side chain shortened by two methylene bridges (-CH2-). CBV is an oxidation product of tetrahydrocannabivarin (THCV, THV).[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
6,6,9-Trimethyl-3-propyl-6H-dibenzo[b,d]pyran-1-ol | |
| Other names
6,6,9-Trimethyl-3-propyl-6H-benzo[c]chromen-1-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| MeSH | cannabivarin |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H22O2 | |
| Molar mass | 282.38 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
It has no double bond isomers nor stereoisomers.
Legal status
It is not scheduled by Convention on Psychotropic Substances.
United States
CBV is not scheduled at the federal level in the United States,[2] but it could be considered an analog (of THC), in which case, sales or possession intended for human consumption could be prosecuted under the Federal Analog Act.
References
- Keith Bailey, Denise Gagné (October 1975). "Distinction of synthetic cannabidiol, cannabichromene, and cannabivarin by GLC using on-column methylation". Journal of Pharmaceutical Sciences. 64 (10): 1719–1720. CiteSeerX 10.1.1.689.8592. doi:10.1002/jps.2600641033. PMID 1185546.
- §1308.11 Schedule I.
See also
- Cannabinoids
- Cannabis
- Medical cannabis
External links
- Erowid Compounds found in Cannabis sativa
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
