Cannabinoid receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system, which is involved in a variety of physiological processes including appetite, pain-sensation, mood, and memory.[1]
| cannabinoid receptor 1 (brain) | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | CNR1 | ||||||
| Alt. symbols | CNR | ||||||
| NCBI gene | 1268 | ||||||
| HGNC | 2159 | ||||||
| OMIM | 114610 | ||||||
| Orthologs | 7273 | ||||||
| RefSeq | NM_033181 | ||||||
| UniProt | P21554 | ||||||
| Other data | |||||||
| Locus | Chr. 6 q14-q15 | ||||||
| |||||||
| cannabinoid receptor 2 (macrophage) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | CNR2 | ||||||
| NCBI gene | 1269 | ||||||
| HGNC | 2160 | ||||||
| OMIM | 605051 | ||||||
| Orthologs | 1389 | ||||||
| RefSeq | NM_001841 | ||||||
| UniProt | P34972 | ||||||
| Other data | |||||||
| Locus | Chr. 1 p | ||||||
| |||||||
| Part of a series on |
| Cannabis |
|---|
 |
|
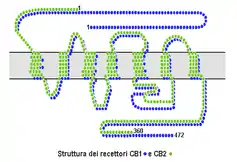
Cannabinoid receptors are of a class of cell membrane receptors in the G protein-coupled receptor superfamily.[2][3][4] As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains.[5] Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids, produced by the mammillary body; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic, such as fat soluble compounds.
There are currently two known subtypes of cannabinoid receptors, termed CB1 and CB2.[6][7] The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system and in hematopoietic cells,[8] however further research has found the existence of these receptors in parts of the brain as well.[9] Mounting evidence suggests that there are novel cannabinoid receptors[10] that is, non-CB1 and non-CB2, which are expressed in endothelial cells and in the CNS. In 2007, the binding of several cannabinoids to the G protein-coupled receptor GPR55 in the brain was described.[11]
The protein sequences of CB1 and CB2 receptors are about 44% similar.[12][13] When only the transmembrane regions of the receptors are considered, amino acid similarity between the two receptor subtypes is approximately 68%.[5] In addition, minor variations in each receptor have been identified. Cannabinoids bind reversibly and stereo-selectively to the cannabinoid receptors. Subtype selective cannabinoids have been developed which theoretically may have advantages for treatment of certain diseases such as obesity.[14]
It appears that cannabinoid receptors are unique to the phylum Chordata and, as such, they have a rather restricted phylogenetic distribution in the animal kingdom. However, enzymes involved in biosynthesis/inactivation of endocannabinoids and endocannabinoid signalling in general (involving targets other than CB1/2-type receptors) occur throughout the animal kingdom.[15] Although the cannabinoid receptors are unique to Chordates, other organisms are still able to process the endocannabinoids through other techniques.
Discovery
The existence of cannabinoid receptors in the brain was discovered from in vitro studies in the 1980s, with the receptor designated as the cannabinoid receptor type 1 or CB1.[16][17] The DNA sequence that encodes a G-protein-coupled cannabinoid receptor in the human brain was identified and cloned in 1990.[18][19] These discoveries led to determination in 1993 of a second brain cannabinoid receptor named cannabinoid receptor type 2 or CB2.[17]
A neurotransmitter for a possible endocannabinoid system in the brain and peripheral nervous system, anandamide (from 'ananda', Sanskrit for 'bliss'), was first characterized in 1992,[20][21][22] followed by discovery of other fatty acid neurotransmitters that behave as endogenous cannabinoids having a low-to-high range of efficacy for stimulating CB1 receptors in the brain and CB2 receptors in the periphery.[17][20]
CB1
Cannabinoid receptor type 1 (CB1) receptors are thought to be one of the most widely expressed Gαi protein-coupled receptors in the brain. One mechanism through which they function is endocannabinoid-mediated depolarization-induced suppression of inhibition, a very common form of retrograde signaling, in which the depolarization of a single neuron induces a reduction in GABA-mediated neurotransmission. Endocannabinoids released from the depolarized post-synaptic neuron bind to CB1 receptors in the pre-synaptic neuron and cause a reduction in GABA release due to limited presynaptic calcium ions entry.
They are also found in other parts of the body. For instance, in the liver, activation of the CB1 receptor is known to increase de novo lipogenesis.[23]
CB2
CB2 receptors are mainly expressed on T cells of the immune system, on macrophages and B cells, and in hematopoietic cells. They also have a function in keratinocytes. They are also expressed on peripheral nerve terminals. These receptors play a role in antinociception, or the relief of pain. In the brain, they are mainly expressed by microglial cells, where their role remains unclear. While the most likely cellular targets and executors of the CB2 receptor-mediated effects of endocannabinoids or synthetic agonists are the immune and immune-derived cells (e.g. leukocytes, various populations of T and B lymphocytes, monocytes/macrophages, dendritic cells, mast cells, microglia in the brain, Kupffer cells in the liver, astrocytes, etc.), the number of other potential cellular targets is expanding, now including endothelial and smooth muscle cells, fibroblasts of various origins, cardiomyocytes, and certain neuronal elements of the peripheral or central nervous systems.[8]
Other cannabinoid receptors
The existence of additional cannabinoid receptors has long been suspected, due to the actions of compounds such as abnormal cannabidiol that produce cannabinoid-like effects on blood pressure and inflammation, yet do not activate either CB1 or CB2.[24][25] Recent research strongly supports the hypothesis that the N-arachidonoyl glycine (NAGly) receptor GPR18 is the molecular identity of the abnormal cannabidiol receptor and additionally suggests that NAGly, the endogenous lipid metabolite of anandamide (also known as arachidonoylethanolamide or AEA), initiates directed microglial migration in the CNS through activation of GPR18.[26] Other molecular biology studies have suggested that the orphan receptor GPR55 should in fact be characterised as a cannabinoid receptor, on the basis of sequence homology at the binding site. Subsequent studies showed that GPR55 does indeed respond to cannabinoid ligands.[11][27] This profile as a distinct non-CB1/CB2 receptor that responds to a variety of both endogenous and exogenous cannabinoid ligands, has led some groups to suggest GPR55 should be categorized as the CB3 receptor, and this re-classification may follow in time.[28] However this is complicated by the fact that another possible cannabinoid receptor has been discovered in the hippocampus, although its gene has not yet been cloned,[29] suggesting that there may be at least two more cannabinoid receptors to be discovered, in addition to the two that are already known. GPR119 has been suggested as a fifth possible cannabinoid receptor,[30] while the PPAR family of nuclear hormone receptors can also respond to certain types of cannabinoid.[31]
Signaling
Cannabinoid receptors are activated by cannabinoids, generated naturally inside the body (endocannabinoids) or introduced into the body as cannabis or a related synthetic compound.[12] Similar responses are produced when introduced in alternative methods, only in a more concentrated form than what is naturally occurring.
After the receptor is engaged, multiple intracellular signal transduction pathways are activated. At first, it was thought that cannabinoid receptors mainly inhibited the enzyme adenylate cyclase (and thereby the production of the second messenger molecule cyclic AMP), and positively influenced inwardly rectifying potassium channels (=Kir or IRK).[32] However, a much more complex picture has appeared in different cell types, implicating other potassium ion channels, calcium channels, protein kinase A and C, Raf-1, ERK, JNK, p38, c-fos, c-jun and many more.[32] For example, in human primary leukocytes CB2 displays a complex signalling profile, activating adenylate cyclase via stimulatory Gαs alongside the classical Gαi signalling, and induces ERK, p38 and pCREB pathways.[33]
Separation between the therapeutically undesirable psychotropic effects, and the clinically desirable ones, however, has not been reported with agonists that bind to cannabinoid receptors. THC, as well as the two major endogenous compounds identified so far that bind to the cannabinoid receptors —anandamide and 2-arachidonylglycerol (2-AG)— produce most of their effects by binding to both the CB1 and CB2 cannabinoid receptors. While the effects mediated by CB1, mostly in the central nervous system, have been thoroughly investigated, those mediated by CB2 are not equally well defined.
Prenatal cannabis exposure (PCE) has been shown to perturb the fetal endogenous cannabinoid signaling system. This perturbation has not been shown to directly affect neurodevelopment nor cause lifelong cognitive, behavioral, or functional abnormalities, but it may predispose offspring to abnormalities in cognition and altered emotionality from post-natal factors.[34] Additionally, PCE may alter the wiring of brain circuitry in foetal development and cause significant molecular modifications to neurodevelopmental programs that may lead to neurophysiological disorders and behavioural abnormalities.[35]
Cannabinoid treatments
Synthetic tetrahydrocannabinol (THC) is prescribed under the INN dronabinol or the brand name Marinol, to treat vomiting and for enhancement of appetite, mainly in people with AIDS as well as for refractory nausea and vomiting in people undergoing chemotherapy.[36] Use of synthetic THC is becoming more common as the known benefits become more prominent within the medical industry. THC is also an active ingredient in nabiximols, a specific extract of Cannabis that was approved as a botanical drug in the United Kingdom in 2010 as a mouth spray for people with multiple sclerosis to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms.[37]
Ligands
Binding affinity and selectivity of cannabinoid ligands
| CB1 affinity (Ki) | Efficacy towards CB1 | CB2 affinity (Ki) | Efficacy towards CB2 | Type | References | |
|---|---|---|---|---|---|---|
| Anandamide | 78nM | Partial agonist | 370nM | ? | Endogenous | |
| N-Arachidonoyl dopamine | ? | Agonist | ? | ? | Endogenous | |
| 2-Arachidonoylglycerol | ? | Full agonist | ? | ? | Endogenous | |
| 2-Arachidonyl glyceryl ether | 21 nM | Full agonist | 480nM | Full agonist | Endogenous | |
| Δ-9-Tetrahydrocannabinol | 10nM | Partial agonist | 24nM | Partial agonist | Phytogenic | [38] |
| EGCG | 33.6μM | Agonist | >50μM | ? | Phytogenic | |
| Yangonin | 0.72 μM | ? | > 10 μM | ? | Phytogenic | [39] |
| AM-1221 | 52.3nM | Agonist | 0.28nM | Agonist | Synthetic | [40] |
| AM-1235 | 1.5nM | Agonist | 20.4nM | Agonist | Synthetic | [41] |
| AM-2232 | 0.28nM | Agonist | 1.48nM | Agonist | Synthetic | [41] |
| UR-144 | 150nM | Full agonist | 1.8nM | Full agonist | Synthetic | [42] |
| JWH-007 | 9.0nM | Agonist | 2.94nM | Agonist | Synthetic | [43] |
| JWH-015 | 383nM | Agonist | 13.8nM | Agonist | Synthetic | [43] |
| JWH-018 | 9.00 ± 5.00 nM | Full agonist | 2.94 ± 2.65 nM | Full agonist | Synthetic | [43] |
See also
References
- Aizpurua-Olaizola O, Elezgarai I, Rico-Barrio I, Zarandona I, Etxebarria N, Usobiaga A (January 2017). "Targeting the endocannabinoid system: future therapeutic strategies". Drug Discovery Today. 22 (1): 105–110. doi:10.1016/j.drudis.2016.08.005. PMID 27554802.
- Howlett AC (August 2002). "The cannabinoid receptors". Prostaglandins & Other Lipid Mediators. 68–69: 619–31. doi:10.1016/S0090-6980(02)00060-6. PMID 12432948.
- Mackie K (May 2008). "Cannabinoid receptors: where they are and what they do". Journal of Neuroendocrinology. 20 Suppl 1: 10–4. doi:10.1111/j.1365-2826.2008.01671.x. PMID 18426493. S2CID 20161611.
- Graham ES, Ashton JC, Glass M (January 2009). "Cannabinoid Receptors: A brief history and what not". Frontiers in Bioscience. 14 (14): 944–57. doi:10.2741/3288. PMID 19273110.
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, et al. (August 1995). "Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations". European Journal of Biochemistry. 232 (1): 54–61. doi:10.1111/j.1432-1033.1995.tb20780.x. PMID 7556170.
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (August 1990). "Structure of a cannabinoid receptor and functional expression of the cloned cDNA". Nature. 346 (6284): 561–4. Bibcode:1990Natur.346..561M. doi:10.1038/346561a0. PMID 2165569. S2CID 4356509.
- Gérard CM, Mollereau C, Vassart G, Parmentier M (October 1991). "Molecular cloning of a human cannabinoid receptor which is also expressed in testis". The Biochemical Journal. 279 (Pt 1): 129–34. doi:10.1042/bj2790129. PMC 1151556. PMID 1718258.
- Pacher P, Mechoulam R (April 2011). "Is lipid signaling through cannabinoid 2 receptors part of a protective system?". Progress in Lipid Research. 50 (2): 193–211. doi:10.1016/j.plipres.2011.01.001. PMC 3062638. PMID 21295074.
- Jordan CJ, Xi ZX (March 2019). "Progress in brain cannabinoid CB2 receptor research: From genes to behavior". Neuroscience and Biobehavioral Reviews. 98: 208–220. doi:10.1016/j.neubiorev.2018.12.026. PMC 6401261. PMID 30611802.
- Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo FM, et al. (May 2005). "Evidence for novel cannabinoid receptors". Pharmacology & Therapeutics. 106 (2): 133–45. doi:10.1016/j.pharmthera.2004.11.005. PMID 15866316.
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. (December 2007). "The orphan receptor GPR55 is a novel cannabinoid receptor". British Journal of Pharmacology. 152 (7): 1092–101. doi:10.1038/sj.bjp.0707460. PMC 2095107. PMID 17876302.
- Latek D, Kolinski M, Ghoshdastider U, Debinski A, Bombolewski R, Plazinska A, et al. (September 2011). "Modeling of ligand binding to G protein coupled receptors: cannabinoid CB1, CB2 and adrenergic β 2 AR". Journal of Molecular Modeling. 17 (9): 2353–66. doi:10.1007/s00894-011-0986-7. PMID 21365223. S2CID 28365397.
- Munro S, Thomas KL, Abu-Shaar M (September 1993). "Molecular characterization of a peripheral receptor for cannabinoids". Nature. 365 (6441): 61–5. Bibcode:1993Natur.365...61M. doi:10.1038/365061a0. PMID 7689702. S2CID 4349125.
- Kyrou I, Valsamakis G, Tsigos C (November 2006). "The endocannabinoid system as a target for the treatment of visceral obesity and metabolic syndrome". Annals of the New York Academy of Sciences. 1083 (1): 270–305. Bibcode:2006NYASA1083..270K. doi:10.1196/annals.1367.024. PMID 17148745. S2CID 23486551.
- Elphick MR (December 2012). "The evolution and comparative neurobiology of endocannabinoid signalling". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 367 (1607): 3201–15. doi:10.1098/rstb.2011.0394. PMC 3481536. PMID 23108540.
- Elphick MR, Egertová M (March 2001). "The neurobiology and evolution of cannabinoid signalling". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 356 (1407): 381–408. doi:10.1098/rstb.2000.0787. PMC 1088434. PMID 11316486.
- Pertwee RG (January 2006). "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology (Review). 147 (Suppl 1): S163–71. doi:10.1038/sj.bjp.0706406. PMC 1760722. PMID 16402100.
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (August 1990). "Structure of a cannabinoid receptor and functional expression of the cloned cDNA". Nature. 346 (6284): 561–4. Bibcode:1990Natur.346..561M. doi:10.1038/346561a0. PMID 2165569. S2CID 4356509.
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. (June 2002). "International Union of Pharmacology. XXVII. Classification of cannabinoid receptors". Pharmacological Reviews (Review). 54 (2): 161–202. doi:10.1124/pr.54.2.161. PMID 12037135. S2CID 8259002.
- Mechoulam R, Fride E (1995). "The unpaved road to the endogenous brain cannabinoid ligands, the anandamides". In Pertwee RG (ed.). Cannabinoid receptors (Review). Boston: Academic Press. pp. 233–258. ISBN 978-0-12-551460-6.
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. (December 1992). "Isolation and structure of a brain constituent that binds to the cannabinoid receptor". Science. 258 (5090): 1946–9. Bibcode:1992Sci...258.1946D. doi:10.1126/science.1470919. PMID 1470919.
- Hanus LO (August 2007). "Discovery and isolation of anandamide and other endocannabinoids". Chemistry & Biodiversity. 4 (8): 1828–41. doi:10.1002/cbdv.200790154. PMID 17712821. S2CID 745528.
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, et al. (May 2005). "Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity". The Journal of Clinical Investigation. 115 (5): 1298–305. doi:10.1172/JCI23057. PMC 1087161. PMID 15864349.
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. (November 1999). "Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors". Proceedings of the National Academy of Sciences of the United States of America. 96 (24): 14136–41. Bibcode:1999PNAS...9614136J. doi:10.1073/pnas.96.24.14136. PMC 24203. PMID 10570211.
- McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA (February 2008). "Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2". Molecular Pharmacology. 73 (2): 441–50. doi:10.1124/mol.107.041863. PMID 17965195. S2CID 15182303.
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, Bradshaw HB (March 2010). "N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor". BMC Neuroscience. 11: 44. doi:10.1186/1471-2202-11-44. PMC 2865488. PMID 20346144.
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. (November 2007). "The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects". British Journal of Pharmacology. 152 (5): 825–31. doi:10.1038/sj.bjp.0707419. PMC 2190033. PMID 17704827.
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. (March 2006). "Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents". Cell Metabolism. 3 (3): 167–75. doi:10.1016/j.cmet.2006.02.004. PMID 16517404.
- de Fonseca FR, Schneider M (June 2008). "The endogenous cannabinoid system and drug addiction: 20 years after the discovery of the CB1 receptor" (PDF). Addiction Biology. 13 (2): 143–6. doi:10.1111/j.1369-1600.2008.00116.x. PMID 18482429. S2CID 205400322. Archived from the original (PDF) on 2011-07-18.
- Brown AJ (November 2007). "Novel cannabinoid receptors". British Journal of Pharmacology. 152 (5): 567–75. doi:10.1038/sj.bjp.0707481. PMC 2190013. PMID 17906678.
- O'Sullivan SE (June 2016). "An update on PPAR activation by cannabinoids". British Journal of Pharmacology. 173 (12): 1899–910. doi:10.1111/bph.13497. PMC 4882496. PMID 27077495.
- Demuth DG, Molleman A (January 2006). "Cannabinoid signalling". Life Sciences. 78 (6): 549–63. doi:10.1016/j.lfs.2005.05.055. PMID 16109430.
- Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL (October 2019). "Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes". ACS Pharmacology & Translational Science. 2 (6): 414–428. doi:10.1021/acsptsci.9b00049. PMC 7088898. PMID 32259074.
- Richardson KA, Hester AK, McLemore GL (2016). "Prenatal cannabis exposure - The "first hit" to the endocannabinoid system". review. Neurotoxicology and Teratology. 58: 5–14. doi:10.1016/j.ntt.2016.08.003. PMID 27567698.
- Calvigioni D, Hurd YL, Harkany T, Keimpema E (October 2014). "Neuronal substrates and functional consequences of prenatal cannabis exposure". review. European Child & Adolescent Psychiatry. 23 (10): 931–41. doi:10.1007/s00787-014-0550-y. PMC 4459494. PMID 24793873.
- Badowski ME (September 2017). "A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics". Cancer Chemotherapy and Pharmacology. 80 (3): 441–449. doi:10.1007/s00280-017-3387-5. PMC 5573753. PMID 28780725.
- "Sativex Oromucosal Spray - Summary of Product Characteristics". UK Electronic Medicines Compendium. March 2015. Archived from the original on 2016-08-22. Retrieved 2017-10-09.
- "PDSP Database - UNC". Archived from the original on 8 November 2013. Retrieved 11 June 2013.
- Ligresti A, Villano R, Allarà M, Ujváry I, Di Marzo V (August 2012). "Kavalactones and the endocannabinoid system: the plant-derived yangonin is a novel CB₁ receptor ligand". Pharmacological Research. 66 (2): 163–9. doi:10.1016/j.phrs.2012.04.003. PMID 22525682.
- WO patent 200128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- US patent 7241799, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2007-07-10
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, et al. (August 2000). "Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding". Drug and Alcohol Dependence. 60 (2): 133–40. doi:10.1016/S0376-8716(99)00152-0. PMID 10940540.
External links
- Cannabinoid+Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)