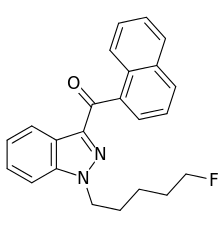
THJ-2201
THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug.[1][2][3][4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H21FN2O |
| Molar mass | 360.432 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It is a structural analog of AM-2201 in which the central indole ring has been replaced by indazole.[5]
Pharmacology
THJ-2201 acts as a full agonist with a binding affinity of 1.34nM at CB1 and 1.32nM at CB2 cannabinoid receptors.[6]
Side effects
THJ-2201 has been linked to at least one hospitalization and death due to its use.[7]
Legal status
Because of the hazards associated with recreational use of this compound,[8] it is classified as a Schedule I controlled substance in the United States.[9]
See also
References
- Diao X, Wohlfarth A, Pang S, Scheidweiler KB, Huestis MA (January 2016). "High-Resolution Mass Spectrometry for Characterizing the Metabolism of Synthetic Cannabinoid THJ-018 and Its 5-Fluoro Analog THJ-2201 after Incubation in Human Hepatocytes". Clinical Chemistry. 62 (1): 157–69. doi:10.1373/clinchem.2015.243535. PMID 26430074.
- Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Morzherin Y, Shafran Y (September 2014). "3-Naphthoylindazoles and 2-naphthoylbenzoimidazoles as novel chemical groups of synthetic cannabinoids: chemical structure elucidation, analytical characteristics and identification of the first representatives in smoke mixtures". Forensic Science International. 242: 72–80. doi:10.1016/j.forsciint.2014.06.022. PMID 25036783.
- Nahoko Uchiyama; Yoshihiko Shimokawa; Maiko Kawamura; Ruri Kikura-Hanajiri; Takashi Hakamatsuka (August 2014). "Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products". Forensic Toxicology. 32 (2): 266–281. doi:10.1007/s11419-014-0238-5. S2CID 11873421.
- Diao X, Scheidweiler KB, Wohlfarth A, Zhu M, Pang S, Huestis MA (2016). "Strategies to distinguish new synthetic cannabinoid FUBIMINA (BIM-2201) intake from its isomer THJ-2201: metabolism of FUBIMINA in human hepatocytes". Forensic Toxicology. 34 (2): 256–267. doi:10.1007/s11419-016-0312-2. PMC 4971051. PMID 27547265.
- "THJ-2201". Cayman Chemical. Retrieved 21 July 2015.
- Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE (1 July 2016). "Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice". Forensic Toxicology. 34 (2): 329–343. doi:10.1007/s11419-016-0320-2. PMC 4929166. PMID 27429655.
- Trecki J, Gerona RR, Schwartz MD (July 2015). "Synthetic Cannabinoid-Related Illnesses and Deaths". The New England Journal of Medicine. 373 (2): 103–7. doi:10.1056/NEJMp1505328. PMID 26154784.
- Drug and Chemical Evaluation Section, Office of Diversion Control, Drug Enforcement Administration (December 2014). "N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3- carboxamide (AB-CHMINACA), N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H- indazole-3-carboxamide (AB-PINACA) and [1-(5-fluoropentyl)-1H-indazol-3- yl](naphthalen-1-yl)methanone (THJ-2201): Background Information and Evaluation of 'Three Factor Analysis' (Factors 4, 5, and 6) for Temporary Scheduling" (PDF). Cite journal requires
|journal=(help)CS1 maint: multiple names: authors list (link) - "Schedules of controlled substances: temporary placement of three synthetic cannabinoids into schedule I. Final order". Federal Register. 80 (20): 5042–7. January 2015. PMID 25730924.
- "Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz - BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)". Retrieved 9 July 2015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.