Clobetasone
Clobetasone (INN[1]) is a corticosteroid used in dermatology, for treating such skin inflammation as seen in eczema, psoriasis and other forms of dermatitis, and ophthalmology. Topical clobetasone butyrate has shown minimal suppression of the hypothalamic–pituitary–adrenal axis.[2]
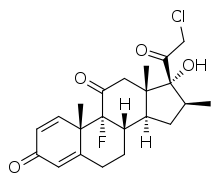 | |
| Clinical data | |
|---|---|
| Trade names | Eumovate |
| Other names | (8S,9R,10S,13S,14S,16S,17R)-17-(2-Chloroacetyl)-9-fluoro-17-hydroxy-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthrene-3,11-dione |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | topical |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.053.576 |
| Chemical and physical data | |
| Formula | C22H26ClFO4 |
| Molar mass | 408.89 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It is available as clobetasone butyrate under the brand names Eumosone or Eumovate[3] both manufactured by GlaxoSmithKline.
Trimovate also contains oxytetracycline, an antibiotic, and nystatin, an antifungal.[4]
Uses
In dermatology, topical clobetasone butyrate helps to reduce the itchiness and erythema associated with eczema and dermatitis.[5]
In ophthalmology, clobetasone butyrate 0.1% eye drops have been shown to be safe and effective in the treatment of dry eyes in Sjögren syndrome. Sjögren syndrome is an autoimmune disorder that affects the moisture producing glands of the body causing many symptoms including dry eyes.[6] When compared to other corticosteroid eye drops; clobetasone butyrate showed only minimal rises in intraocular pressure. Increased pressure within the eye can lead to glaucoma.[7][8][9]
Adverse effects
Side effects associated with clobetasone cream and ointment include: burning, irritation, itching, thinning of the skin, and changes in skin color.[5][10]
References
- "United Nations Statistics Division - Classifications Registry - Alphabetical index for HS 2002 Entries starting with 'C' (page 310 of 422)". Archived from the original on 2011-06-08. Retrieved 2007-12-11.
- Munro DD, Wilson L (September 1975). "Clobetasone butyrate, a new topical corticosteroid: clinical activity and effects on pituitary-adrenal axis function and model of epidermal atrophy". British Medical Journal. 3 (5984): 626–8. doi:10.1136/bmj.3.5984.626. PMC 1674413. PMID 1164639.
- "Eumovate cream/ointment". NetDoctor.co.uk. 2017-07-05.
- "Trimovate". Electronic Medicines Compendium (EMC). Datapharm Ltd.
- "Euvomate Medication Guide". GlaxoSmithKline. Archived from the original on 30 January 2014.
- Aragona P, Spinella R, Rania L, Postorino E, Sommario MS, Roszkowska AM, Puzzolo D (2013). "Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren syndrome". European Journal of Ophthalmology. 23 (3): 368–76. doi:10.5301/ejo.5000229. PMID 23225089. S2CID 20730843.
- Ramsell TG, Bartholomew RS, Walker SR (January 1980). "Clinical evaluation of clobetasone butyrate: a comparative study of its effects in postoperative inflammation and on intraocular pressure". The British Journal of Ophthalmology. 64 (1): 43–5. doi:10.1136/bjo.64.1.43. PMC 1039346. PMID 6986899.
- Wilhelmus KR, Hunter PA, Rice NS (October 1981). "Equivalence of topical clobetasone and dexamethasone in experimental corneal allograft rejection". The British Journal of Ophthalmology. 65 (10): 699–701. doi:10.1136/bjo.65.10.699. PMC 1039641. PMID 7032579.
- Eilon LA, Walker SR (September 1981). "Clinical evaluation of clobetasone butyrate eye drops in the treatment of anterior uveitis and its effects on intraocular pressure". The British Journal of Ophthalmology. 65 (9): 644–7. doi:10.1136/bjo.65.9.644. PMC 1039614. PMID 7028089.
- "Clobetason Butyrate". WebMD.
