Telapristone
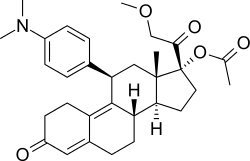
Telapristone (INN), as telapristone acetate (proposed brand names Proellex, Progenta; former code name CDB-4124), is a synthetic, steroidal selective progesterone receptor modulator (SPRM) related to mifepristone which is under development by Repros Therapeutics for the treatment of breast cancer, endometriosis, and uterine fibroids.[1][2] It was originally developed by the National Institutes of Health (NIH), and, as of 2017, is in phase II clinical trials for the aforementioned indications.[1] In addition to its activity as an SPRM, the drug also has some antiglucocorticoid activity.[3]
 | |
| Clinical data | |
|---|---|
| Other names | CDB-4124; Proellex; Progenta; 17β-(Acetyloxy)-11β-[4-(dimethylamino)phenyl]-17α-(2-methoxyacetyl)estra-4,9-dien-3-one |
| Drug class | Selective progesterone receptor modulator |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C31H39NO5 |
| Molar mass | 505.655 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
See also
References
- http://adisinsight.springer.com/drugs/800014404
- Attardi BJ, Burgenson J, Hild SA, Reel JR (2004). "In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone". J. Steroid Biochem. Mol. Biol. 88 (3): 277–88. doi:10.1016/j.jsbmb.2003.12.004. PMID 15120421. S2CID 23958876.
- Whitaker LH, Williams AR, Critchley HO (2014). "Selective progesterone receptor modulators". Curr. Opin. Obstet. Gynecol. 26 (4): 237–42. doi:10.1097/GCO.0000000000000082. PMID 24950125. S2CID 37474964.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.