Fish jaw
Most bony fishes have two sets of jaws made mainly of bone. The primary oral jaws open and close the mouth, and a second set of pharyngeal jaws are positioned at the back of the throat. The oral jaws are used to capture and manipulate prey by biting and crushing. The pharyngeal jaws, so-called because they are positioned within the pharynx, are used to further process the food and move it from the mouth to the stomach.[2][3]
.tiff.png.webp)
.tiff.png.webp)
Cartilaginous fishes, such as sharks and rays, have one set of oral jaws made mainly of cartilage. They do not have pharyngeal jaws. Generally jaws are articulated and oppose vertically, comprising an upper jaw and a lower jaw and can bear numerous ordered teeth. Cartilaginous fishes grow multiple sets (polyphyodont) and replace teeth as they wear by moving new teeth laterally from the medial jaw surface in a conveyor-belt fashion. Teeth are replaced multiple times also in most bony fishes, but unlike cartilaginous fishes, the new tooth erupts only after the old one has fallen out.
Jaws probably originated in the pharyngeal arches supporting the gills of jawless fish. The earliest jaws appeared in now extinct placoderms and spiny sharks during the Silurian, about 430 million years ago. The original selective advantage offered by the jaw was probably not related to feeding, but to increased respiration efficiency—the jaws were used in the buccal pump to pump water across the gills. The familiar use of jaws for feeding would then have developed as a secondary function before becoming the primary function in many vertebrates. All vertebrate jaws, including the human jaw, evolved from early fish jaws. The appearance of the early vertebrate jaw has been described as "perhaps the most profound and radical evolutionary step in the vertebrate history".[4][5] Fish without jaws had more difficulty surviving than fish with jaws, and most jawless fish became extinct.
Jaws use linkage mechanisms. These linkages can be especially common and complex in the head of bony fishes, such as wrasses, which have evolved many specialized feeding mechanisms. Especially advanced are the linkage mechanisms of jaw protrusion. For suction feeding a system of linked four-bar linkages is responsible for the coordinated opening of the mouth and the three-dimensional expansion of the buccal cavity. The four-bar linkage is also responsible for protrusion of the premaxilla,[6] leading to three main four-bar linkage systems to generally describe the lateral and anterior expansion of the buccal cavity in fishes.[6][7] The most thorough overview of the different types of linkages in animals has been provided by M. Muller,[8] who also designed a new classification system, which is especially well suited for biological systems.
Skull
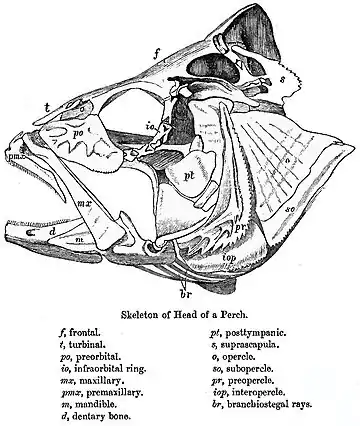
The skull of fishes is formed from a series of loosely connected bones. Lampreys and sharks only possess a cartilaginous endocranium, with both the upper and lower jaws being separate elements. Bony fishes have additional dermal bone, forming a more or less coherent skull roof in lungfish and holost fish.
The simpler structure is found in jawless fish, in which the cranium is represented by a trough-like basket of cartilaginous elements only partially enclosing the brain, and associated with the capsules for the inner ears and the single nostril. Distinctively, these fish have no jaws.[9]
Cartilaginous fish, such as sharks, also have simple skulls. The cranium is a single structure forming a case around the brain, enclosing the lower surface and the sides, but always at least partially open at the top as a large fontanelle. The most anterior part of the cranium includes a forward plate of cartilage, the rostrum, and capsules to enclose the olfactory organs. Behind these are the orbits, and then an additional pair of capsules enclosing the structure of the inner ear. Finally, the skull tapers towards the rear, where the foramen magnum lies immediately above a single condyle, articulating with the first vertebra. There are, in addition, at various points throughout the cranium, smaller foramina for the cranial nerves. The jaws consist of separate hoops of cartilage, almost always distinct from the cranium proper.[9]
In ray-finned fishes, there has also been considerable modification from the primitive pattern. The roof of the skull is generally well formed, and although the exact relationship of its bones to those of tetrapods is unclear, they are usually given similar names for convenience. Other elements of the skull, however, may be reduced; there is little cheek region behind the enlarged orbits, and little, if any bone in between them. The upper jaw is often formed largely from the premaxilla, with the maxilla itself located further back, and an additional bone, the symplectic, linking the jaw to the rest of the cranium.[9]
Although the skulls of fossil lobe-finned fish resemble those of the early tetrapods, the same cannot be said of those of the living lungfishes. The skull roof is not fully formed, and consists of multiple, somewhat irregularly shaped bones with no direct relationship to those of tetrapods. The upper jaw is formed from the pterygoids and vomers alone, all of which bear teeth. Much of the skull is formed from cartilage, and its overall structure is reduced.[9]
Oral jaws
Lower
In vertebrates, the lower jaw (mandible or jawbone)[10] is a bone forming the skull with the cranium. In lobe-finned fishes and the early fossil tetrapods, the bone homologous to the mandible of mammals is merely the largest of several bones in the lower jaw. It is referred to as the dentary bone, and forms the body of the outer surface of the jaw. It is bordered below by a number of splenial bones, while the angle of the jaw is formed by a lower angular bone and a suprangular bone just above it. The inner surface of the jaw is lined by a prearticular bone, while the articular bone forms the articulation with the skull proper. Finally a set of three narrow coronoid bones lie above the prearticular bone. As the name implies, the majority of the teeth are attached to the dentary, but there are commonly also teeth on the coronoid bones, and sometimes on the prearticular as well.[11]
This complex primitive pattern has, however, been simplified to various degrees in the great majority of vertebrates, as bones have either fused or vanished entirely. In teleosts, only the dentary, articular, and angular bones remain.[11] Cartilagenous fish, such as sharks, do not have any of the bones found in the lower jaw of other vertebrates. Instead, their lower jaw is composed of a cartilagenous structure homologous with the Meckel's cartilage of other groups. This also remains a significant element of the jaw in some primitive bony fish, such as sturgeons.[11]
Upper
The upper jaw, or maxilla[12][13] is a fusion of two bones along the palatal fissure that form the upper jaw. This is similar to the mandible (lower jaw), which is also a fusion of two halves at the mandibular symphysis. In bony fish, the maxilla is called the "upper maxilla," with the mandible being the "lower maxilla". The alveolar process of the maxilla holds the upper teeth, and is referred to as the maxillary arch. In most vertebrates, the foremost part of the upper jaw, to which the incisors are attached in mammals consists of a separate pair of bones, the premaxillae. In bony fish, both maxilla and premaxilla are relatively plate-like bones, forming only the sides of the upper jaw, and part of the face, with the premaxilla also forming the lower boundary of the nostrils.[14] Cartilaginous fish, such as sharks and rays also lack a true maxilla. Their upper jaw is instead formed from a cartilagenous bar that is not homologous with the bone found in other vertebrates.[14]
Some fish have permanently protruding upper jawbones called rostrums. Billfish (marlin, swordfish and sailfish) use rostrums (bills) to slash and stun prey. Paddlefish, goblin sharks and hammerhead sharks have rostrums packed with electroreceptors which signal the presence of prey by detecting weak electrical fields. Sawsharks and the critically endangered sawfish have rostrums (saws) which are both electro-sensitive and used for slashing.[15] The rostrums extend ventrally in front of the fish. In the case of hammerheads the rostrum (hammer) extends both ventrally and laterally (sideways).
- Fish with rostrums (extended upper jawbones)

 The paddlefish has a rostrum packed with electroreceptors
The paddlefish has a rostrum packed with electroreceptors Sawfish have an electro-sensitive rostrum (saw) which is also used to slash at prey
Sawfish have an electro-sensitive rostrum (saw) which is also used to slash at prey
Jaw protrusion
Teleosts have a movable premaxilla (a bone at the tip of the upper jaw) and corresponding modifications in the jaw musculature which make it possible for them to protrude their jaws outwards from the mouth. This is of great advantage, enabling them to grab prey and draw it into the mouth. In more derived teleosts, the enlarged premaxilla is the main tooth-bearing bone, and the maxilla, which is attached to the lower jaw, acts as a lever, pushing and pulling the premaxilla as the mouth is opened and closed. These protrusible jaws are evolutionary novelties in teleosts that evolved independently at least five times.[16]
The premaxilla is unattached to the neurocranium (braincase); it plays a role in protruding the mouth and creating a circular opening. This lowers the pressure inside the mouth, sucking the prey inside. The lower jaw and maxilla (main upper fixed bone of the jaw) are then pulled back to close the mouth, and the fish is able to grasp the prey. By contrast, mere closure of the jaws would risk pushing food out of the mouth. In more advanced teleosts, the premaxilla is enlarged and has teeth, while the maxilla is toothless. The maxilla functions to push both the premaxilla and the lower jaw forward. To open the mouth, an adductor muscle pulls back the top of the maxilla, pushing the lower jaw forward. In addition, the maxilla rotates slightly, which pushes forward a bony process that interlocks with the premaxilla.[17]
Teleosts achieve this jaw protrusion using one of four different mechanisms involving the ligamentous linkages within the skull.[18]


- Mandibular depression mechanism: The depression of the lower jaw (mandible) pulls or pushes the premaxilla into protrusion via force transmission through ligaments and tendons connected to the upper jaws (e.g. Cyprinus, Labrus).[18] This is the most commonly used mechanism.
- Twisting maxilla mechanism: The depression of the mandible causes the maxilla to twist about the longitudinal axis resulting in the protrusion of the premaxilla (e.g. Mugil).[18]
- Decoupled mechanism: Protrusion of the premaxilla is accomplished through elevation of the neurocranium causing the premaxilla to move anteriorly. Movements of the neurocranium are not coupled with the kinematics of the upper jaw (e.g. Spathodus erythrodon),[18][19] allowing for more versatility and modularity of the jaws during prey capture and manipulation.
- Suspensorial abduction mechanism: The lateral expansion of the suspensorium (a combination of the palatine, pterygoid series, and quadrate bones) pulls on a ligament which causes the premaxilla to protrude anteriorly (e.g. Petrotilapia tridentiger).[18][19]
Some teleosts use more than one of these mechanisms (e.g. Petrotilapia).[18]
Wrasses have become a primary study species in fish-feeding biomechanics due to their jaw structure. They have protractile mouths, usually with separate jaw teeth that jut outwards.[20] Many species can be readily recognized by their thick lips, the inside of which is sometimes curiously folded, a peculiarity which gave rise the German name of "lip-fishes" (Lippfische).[21]
The nasal and mandibular bones are connected at their posterior ends to the rigid neurocranium, and the superior and inferior articulations of the maxilla are joined to the anterior tips of these two bones, respectively, creating a loop of 4 rigid bones connected by moving joints. This "four-bar linkage" has the property of allowing numerous arrangements to achieve a given mechanical result (fast jaw protrusion or a forceful bite), thus decoupling morphology from function. The actual morphology of wrasses reflects this, with many lineages displaying different jaw morphology that results in the same functional output in a similar or identical ecological niche.[20]
The most extreme jaw protrusion found in fishes occurs in the slingjaw wrasse, Epibulus insidiator . This fish can extend its jaws up to 65% the length of its head.[22] This species utilizes its quick and extreme jaw protrusion to capture smaller fishes and crustaceans. The genus this species belongs to possess one unique ligament (vomero-interopercular) and two enlarged ligaments (interoperculo-mandibular and premaxilla-maxilla), which along with a few changes to the form of cranial bones, allow it to achieve extreme jaw protrusion.
Pharyngeal jaws
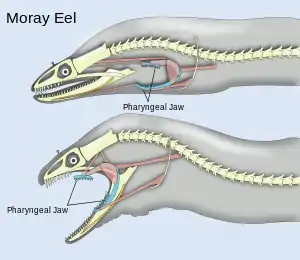
Pharyngeal jaws are a second set of jaws distinct from the primary (oral) jaws. They are contained within the throat, or pharynx, of most bony fish. They are believed to have originated, in a similar way to oral jaws, as a modification of the fifth gill arch which no longer has a respiratory function. The first four arches still function as gills. Unlike the oral jaw, the pharyngeal jaw has no jaw joint, but is supported instead by a sling of muscles.
_Schlundzaehne_1x_Fig_147_(Matschie_et_al._1909).svg.png.webp)
A notable example occurs with the moray eel. The pharyngeal jaws of most fishes are not mobile. The pharyngeal jaws of the moray are highly mobile, perhaps as an adaptation to the constricted nature of the burrows they inhabit which inhibits their ability to swallow as other fishes do by creating a negative pressure in the mouth. Instead, when the moray bites prey, it first bites normally with its oral jaws, capturing the prey. Immediately thereafter, the pharyngeal jaws are brought forward and bite down on the prey to grip it; they then retract, pulling the prey down the moray eel's gullet, allowing it to be swallowed.[23]
All vertebrates have a pharynx, used in both feeding and respiration. The pharynx arises during development through a series of six or more outpocketings called pharyngeal arches on the lateral sides of the head. The pharyngeal arches give rise to a number of different structures in the skeletal, muscular and circulatory systems in a manner which varies across the vertebrates. Pharyngeal arches trace back through chordates to basal deuterostomes who also share endodermal outpocketings of the pharyngeal apparatus. Similar patterns of gene expression can be detected in the developing pharynx of amphioxus and hemichordates. However, the vertebrate pharynx is unique in that it gives rise to endoskeletal support through the contribution of neural crest cells.[24]
Cartilaginous jaws
Cartilaginous fishes (sharks, rays and skates) have cartilaginous jaws. The jaw's surface (in comparison to the vertebrae and gill arches) needs extra strength due to its heavy exposure to physical stress. It has a layer of tiny hexagonal plates called "tesserae", which are crystal blocks of calcium salts arranged as a mosaic.[25] This gives these areas much of the same strength found in the bony tissue found in other animals.
Generally sharks have only one layer of tesserae, but the jaws of large specimens, such as the bull shark, tiger shark, and the great white shark, have two to three layers or more, depending on body size. The jaws of a large great white shark may have up to five layers.[26] In the rostrum (snout), the cartilage can be spongy and flexible to absorb the power of impacts.
In sharks and other extant elasmobranchs the upper jaw is not fused to the cranium, and the lower jaw is articulated with the upper. The arrangement of soft tissue and any additional articulations connecting these elements is collectively known as the jaw suspension. There are several archetypal jaw suspensions: amphistyly, orbitostyly, hyostyly, and euhyostyly. In amphistyly, the palatoquadrate has a postorbital articulation with the chondrocranium from which ligaments primarily suspend it anteriorly. The hyoid articulates with the mandibular arch posteriorly, but it appears to provide little support to the upper and lower jaws. In orbitostyly, the orbital process hinges with the orbital wall and the hyoid provides the majority of suspensory support. In contrast, hyostyly involves an ethmoid articulation between the upper jaw and the cranium, while the hyoid most likely provides vastly more jaw support compared to the anterior ligaments. Finally, in euhyostyly, also known as true hyostyly, the mandibular cartilages lack a ligamentous connection to the cranium. Instead, the hyomandibular cartilages provide the only means of jaw support, while the ceratohyal and basihyal elements articulate with the lower jaw, but are disconnected from the rest of the hyoid.[27][28][29]
Teeth

.jpeg.webp)
Jaws provide a platform in most fishes for simple pointed teeth. Lungfish and chimaera have teeth modified into broad enamel plates with jagged ridges for crushing or grinding. Carp and loach have pharyngeal teeth only. Sea horses, pipefish and adult sturgeon have no teeth of any type. In fish, Hox gene expression regulates mechanisms for tooth initiation.[30][31]
However, sharks continuously produce new teeth throughout their lives via a drastically different mechanism.[32][33][34] Shark teeth form from modified scales near the tongue and move outward on the jaw in rows until they are eventually dislodged.[35] Their scales, called dermal denticles, and teeth are homologous organs.[36]
Shark teeth are embedded in the gums rather than directly affixed to the jaw, and are constantly replaced throughout life. Multiple rows of replacement teeth grow in a groove on the inside of the jaw and steadily moving forward as though on a conveyor belt. Some sharks lose 30,000 or more teeth in their lifetime. The rate of tooth replacement varies from once every 8 to 10 days to several months. In most species, teeth are replaced one at a time as opposed to the simultaneous replacement of an entire row, which is observed in the cookiecutter shark.[37]
Tooth shape depends on the shark's diet: those that feed on mollusks and crustaceans have dense and flattened teeth used for crushing, those that feed on fish have needle-like teeth for gripping, and those that feed on larger prey such as mammals have pointed lower teeth for gripping and triangular upper teeth with serrated edges for cutting. The teeth of plankton-feeders such as the basking shark are small and non-functional.[38]
- Cartilaginous jaws and their teeth
 Jaw reconstruction of the extinct Carcharodon megalodon, 1909
Jaw reconstruction of the extinct Carcharodon megalodon, 1909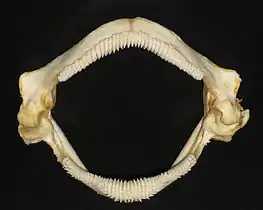 The thornback ray has teeth adapted to feed on crabs, shrimps and small fish.
The thornback ray has teeth adapted to feed on crabs, shrimps and small fish. The shortfin mako shark lunges vertically and tears flesh from prey
The shortfin mako shark lunges vertically and tears flesh from prey Tiger shark teeth are oblique and serrated to saw through flesh
Tiger shark teeth are oblique and serrated to saw through flesh The prickly shark has knife-like teeth with main cusps flanked by lateral cusplets
The prickly shark has knife-like teeth with main cusps flanked by lateral cusplets
Examples
Salmon
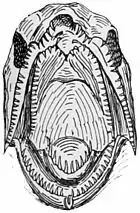

Male salmon often remodel their jaws during spawning runs so they have a pronounced curvature. These hooked jaws are called kypes. The purpose of the kype is not altogether clear, though they can be used to establish dominance by clamping them around the base of the tail (caudal peduncle) of an opponent.[39][40]
Cichlids

Fish jaws, like vertebrates in general, normally show bilateral symmetry. An exception occurs with the parasitic scale-eating cichlid Perissodus microlepis. The jaws of this fish occur in two distinct morphological forms. One morph has its jaw twisted to the left, allowing it to eat scales more readily on its victim's right flank. The other morph has its jaw twisted to the right, which makes it easier to eat scales on its victim's left flank. The relative abundance of the two morphs in populations is regulated by frequency-dependent selection.[41][42][43]
In cichlids generally, the oral and pharyngeal teeth differ with different species in ways that allow them to process different kinds of prey. Primary oral jaws contain teeth which are used to capture and hold food, while pharyngeal jaws have pharyngeal teeth which function as a chewing tool.
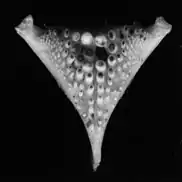
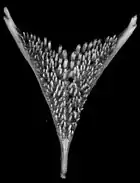
This allows for different nutritional strategies, and because of this, cichlids are able to colonize different habitats. The structural diversity of the lower pharyngeal jaw could be one of the reasons for the occurrence of so many cichlid species. Convergent evolution took place over the course of the cichlid radiation, synchronous with different trophic niches.[44] The pharyngeal jaw apparatus consists of two upper and one single lower plate, all of which have dentations that differ in size and type.[45] The structure of the lower pharynx is often associated with the species of food of the species.[46]
In order to crack shellfish considerable force must be generated, which is why cichlids that feed on molluscs (e.g. the cichlid bass, Crenicichla minuano), have molariform teeth and a strengthened jawbone bone. To grab and bite prey not armoured with shells, predators need conical, bent back teeth.[47] Herbivorous cichlids also have structural differences in their teeth. Cichlids that specialise in algae (e.g. Pseudotropheus) tend to have small conical teeth. Species that feed on pods or seeds require large conical teeth for chewing their food.[48]
Other
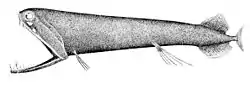
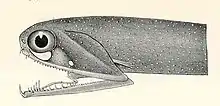
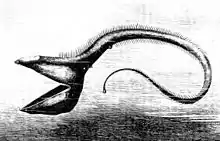
Stoplight loosejaws are small fish found worldwide in the deep sea. Relative to their size they have one of the widest gapes of any fish. The lower jaw has no ethmoid membrane (floor) and is attached only by the hinge and a modified tongue bone. There are several large, fang-like teeth in the front of the jaws, followed by many small barbed teeth. There are several groups of pharyngeal teeth that serve to direct food down the esophagus.[49][50]
Another deep sea fish, the pelican eel, has jaws larger than its body. The jaws are lined with small teeth and are loosely hinged. They open wide enough to swallow a fish larger than the eel itself.
Distichodontidae are a family of fresh water fishes which can be divided into genera with protractile upper jaws which are carnivores, and genera with nonprotractile upper jaws which are herbivores or predators of very small organisms.[51]
Evolution
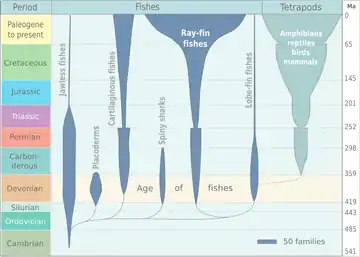
The appearance of the early vertebrate jaw has been described as "a crucial innovation"[53] and "perhaps the most profound and radical evolutionary step in the vertebrate history".[4][5] Fish without jaws had more difficulty surviving than fish with jaws, and most jawless fish became extinct during the Triassic period. However studies of the cyclostomes, the jawless hagfishes and lampreys that did survive, have yielded little insight into the deep remodelling of the vertebrate skull that must have taken place as early jaws evolved.[54][55]
The customary view is that jaws are homologous to the gill arches.[56] In jawless fishes a series of gills opened behind the mouth, and these gills became supported by cartilaginous elements. The first set of these elements surrounded the mouth to form the jaw. The upper portion of the second embryonic arch supporting the gill became the hyomandibular bone of jawed fishes, which supports the skull and therefore links the jaw to the cranium.[57] The hyomandibula is a set of bones found in the hyoid region in most fishes. It usually plays a role in suspending the jaws or the operculum in the case of teleosts.[58]
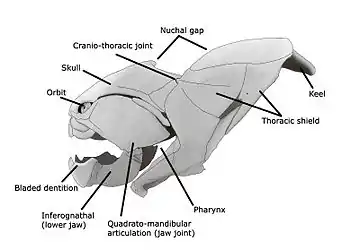


It is now accepted that the precursors of the jawed vertebrates are the long extinct bony (armoured) jawless fish, the so-called ostracoderms.[59][60] The earliest known fish with jaws are the now extinct placoderms[61] and spiny sharks.[62]
Placoderms were a class of fish, heavily armoured at the front of their body, which first appeared in the fossil records during the Silurian about 430 million years ago. Initially they were very successful, diversifying remarkably during the Devonian. They became extinct by the end of that period, about 360 million years ago.[63] Their largest species, Dunkleosteus terrelli, measured up to 10 m (33 ft)[64][65] and weighed 3.6 t (4.0 short tons).[66] It possessed a four bar linkage mechanism for jaw opening that incorporated connections between the skull, the thoracic shield, the lower jaw and the jaw muscles joined together by movable joints.[67][68] This mechanism allowed Dunkleosteus terrelli to achieve a high speed of jaw opening, opening their jaws in 20 milliseconds and completing the whole process in 50-60 milliseconds, comparable to modern fishes that use suction feeding to assist in prey capture.[67] They could also produce high bite forces when closing the jaw, estimated at 6,000 N (1,350 lbf) at the tip and 7,400 N (1,660 lbf) at the blade edge in the largest individuals.[68] The pressures generated in those regions were high enough to puncture or cut through cuticle or dermal armour[67] suggesting that Dunkleosteus terrelli was perfectly adapted to prey on free-swimming, armoured prey like arthropods, ammonites, and other placoderms.[68]
Spiny sharks were another class of fish which appeared also in the fossil records during the Silurian at about the same time as the placoderms. They were smaller than most placoderms, usually under 20 centimetres. Spiny sharks did not diversify as much as placoderms, but survived much longer into the Early Permian about 290 million years ago.[69]
The original selective advantage offered by the jaw may not be related to feeding, but rather to increased respiration efficiency.[70] The jaws were used in the buccal pump still observable in modern fish and amphibians, that uses "breathing with the cheeks" to pump water across the gills of fish or air into the lungs in the case of amphibians. Over evolutionary time the more familiar use of jaws (to humans), in feeding, was selected for and became a very important function in vertebrates. Many teleost fish have substantially modified jaws for suction feeding and jaw protrusion, resulting in highly complex jaws with dozens of bones involved.[71]
Jaws are thought to derive from the pharyngeal arches that support the gills in fish. The two most anterior of these arches are thought to have become the jaw itself (see hyomandibula) and the hyoid arch, which braces the jaw against the braincase and increases mechanical efficiency. While there is no fossil evidence directly to support this theory, it makes sense in light of the numbers of pharyngeal arches that are visible in extant jawed (the Gnathostomes), which have seven arches, and primitive jawless vertebrates (the Agnatha), which have nine.
Meckel's cartilage is a piece of cartilage from which the mandibles (lower jaws) of vertebrates evolved. Originally it was the lower of two cartilages which supported the first gill arch (nearest the front) in early fish. Then it grew longer and stronger, and acquired muscles capable of closing the developing jaw.[72] In early fish and in chondrichthyans (cartilaginous fish such as sharks), Meckel's cartilage continued to be the main component of the lower jaw. But in the adult forms of osteichthyans (bony fish) and their descendants (amphibians, reptiles, birds and mammals) the cartilage was covered in bone – although in their embryos the jaw initially develops as the Meckel's cartilage. In tetrapods the cartilage partially ossifies (changes to bone) at the rear end of the jaw and becomes the articular bone, which forms part of the jaw joint in all tetrapods except mammals.[72]
See also
Notes
- Fraser, G. J.; Hulsey, C. D.; Bloomquist, R. F.; Uyesugi, K.; Manley, N. R.; Streelman, J. T. (2009). "An ancient gene network is co-opted for teeth on old and new jaws". PLOS Biology. 7 (2): e1000031. doi:10.1371/journal.pbio.1000031. PMC 2637924. PMID 19215146.
- Mabuchi, K.; Miya, M.; Azuma, Y.; Nishida, M. (2007). "Independent evolution of the specialized pharyngeal jaw apparatus in cichlid and labrid fishes". BMC Evolutionary Biology. 7 (1): 10. doi:10.1186/1471-2148-7-10. PMC 1797158. PMID 17263894.
- Alfaro, M. E.; Brock, C. D.; Banbury, B. L.; Wainwright, P. C. (2009). "Does evolutionary innovation in pharyngeal jaws lead to rapid lineage diversification in labrid fishes?". BMC Evolutionary Biology. 9 (1): 255. doi:10.1186/1471-2148-9-255. PMC 2779191. PMID 19849854.
- Gai, Z.; Zhu, M. (2012). "The origin of the vertebrate jaw: Intersection between developmental biology-based model and fossil evidence". Chinese Science Bulletin. 57 (30): 3819–3828. doi:10.1007/s11434-012-5372-z.
- Maisey, J. G. (2000). Discovering Fossil Fishes. Westview Press. pp. 1–223. ISBN 978-0-8133-3807-1.
- Westneat, Mark W. (September 1990). "Feeding mechanics of teleost fishes (Labridae; Perciformes): A test of four-bar linkage models". Journal of Morphology. 205 (3): 269–295. doi:10.1002/jmor.1052050304. ISSN 0362-2525. PMID 29865760.
- Olsen, Aaron M.; Camp, Ariel L.; Brainerd, Elizabeth L. (2017-12-13). "The opercular mouth-opening mechanism of largemouth bass functions as a 3D four-bar linkage with three degrees of freedom". The Journal of Experimental Biology. 220 (24): 4612–4623. doi:10.1242/jeb.159079. ISSN 0022-0949. PMID 29237766.
- Muller, M. (1996). "A novel classification of planar four-bar linkages and its application to the mechanical analysis of animal systems" (PDF). Phil. Trans. R. Soc. Lond. B. 351 (1340): 689–720. doi:10.1098/rstb.1996.0065. PMID 8927640.
- Romer & Parsons 1977, pp. 173–177
- The mandible is also in some sources still referred to as the inferior maxillary bone, though this is an outdated term which goes back to at least the 1858 first edition of Gray's Anatomy, if not earlier.
- Romer & Parsons 1977, pp. 244–247
- OED 2nd edition, 1989.
- "maxilla". Merriam-Webster Online Dictionary.
- Romer & Parsons 1977, pp. 217–243
- Wueringer, B. E.; Squire, L., Jr; Kajiura, S. M.; Hart, N. S.; Collin, S. P. (2012). "The function of the sawfish's saw". Current Biology. 22 (5): R150–R151. doi:10.1016/j.cub.2012.01.055. PMID 22401891.
- Westneat, M. W. (2004-11-01). "Evolution of Levers and Linkages in the Feeding Mechanisms of Fishes". Integrative and Comparative Biology. 44 (5): 378–389. doi:10.1093/icb/44.5.378. ISSN 1540-7063. PMID 21676723.
- Benton, Michael (2005). "The Evolution of Fishes After the Devonian". Vertebrate Palaeontology (3rd ed.). John Wiley & Sons. pp. 175–84. ISBN 978-1-4051-4449-0.
- Motta, Philip Jay (1984-02-23). "Mechanics and Functions of Jaw Protrusion in Teleost Fishes: A Review". Copeia. 1984 (1): 1–18. doi:10.2307/1445030. ISSN 0045-8511. JSTOR 1445030.
- LIEM, KAREL F (February 1980). "Adaptive Significance of Intra- and Interspecific Differences in the Feeding Repertoires of Cichlid Fishes". American Zoologist. 20 (1): 295–314. doi:10.1093/icb/20.1.295. ISSN 0003-1569.
- Wainwright, Peter C.; Alfaro, Michael E.; Bolnick, Daniel I.; Hulsey, C. Darrin (2005). "Many-to-One Mapping of Form to Function: A General Principle in Organismal Design?". Integrative and Comparative Biology. 45 (2): 256–262. doi:10.1093/icb/45.2.256. PMID 21676769.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 28 (11th ed.). Cambridge University Press. p. 839.
- Westneat, Wainwright (1989). "Feeding Mechanism of Epibulus insidiator (Labridae; Teleostei): Evolution of a Novel Functional System". Journal of Morphology. 202 (2): 129–150. doi:10.1002/jmor.1052020202. PMID 29865677.
- Mehta, Rita S.; Wainwright, Peter C. (2007-09-06). "Raptorial jaws in the throat help moray eels swallow large prey". Nature. 449 (7158): 79–82. doi:10.1038/nature06062. PMID 17805293.
- Graham, A; Richardson, J (2012). "Developmental and evolutionary origins of the pharyngeal apparatus". EvoDevo. 3 (1): 24. doi:10.1186/2041-9139-3-24. PMC 3564725. PMID 23020903.
- Hamlett, W. C. (1999f). Sharks, Skates and Rays: The Biology of Elasmobranch Fishes. Johns Hopkins University Press. ISBN 978-0-8018-6048-5. OCLC 39217534.
- Martin, R. Aidan. "Skeleton in the Corset". ReefQuest Centre for Shark Research. Retrieved 2009-08-21.
- Wilga, C. D. (2005). "Morphology and evolution of the jaw suspension in lamniform sharks". Journal of Morphology. 265 (1): 102–119. doi:10.1002/jmor.10342. PMID 15880740.
- Wilga, C. D.; Motta, P. J.; Sanford, C. P. (2007). "Evolution and ecology of feeding in elasmobranchs". Integrative and Comparative Biology. 47 (1): 55–69. doi:10.1093/icb/icm029. PMID 21672820.
- Motta, Philip J.; Huber, Daniel R. (2012). "Prey Capture Behavior and Feeding Mechanisms of Elasmobranchs". In Carrier, J. C.; Musick, J. A.; Heithaus, M. R. (eds.). Biology of Sharks and Their Relatives (Second ed.). CRC Press. pp. 153–210. ISBN 978-1-4398-3924-9.
- Fraser GJ, Hulsey CD, Bloomquist RF, Uyesugi K, Manley NR, Streelman JT (February 2009). Jernvall J (ed.). "An Ancient Gene Network Is Co-opted for Teeth on Old and New Jaws". PLOS Biology. 7 (2): e31. doi:10.1371/journal.pbio.1000031. PMC 2637924. PMID 19215146.
- Fraser GJ, Bloomquist RF, Streelman JT (2008). "A periodic pattern generator for dental diversity". BMC Biology. 6: 32. doi:10.1186/1741-7007-6-32. PMC 2496899. PMID 18625062.
- Dave Abbott, Sharks, found here
- Boyne PJ (1970). "Study of the chronologic development and eruption of teeth in elasmobranchs". Journal of Dental Research. 49 (3): 556–60. doi:10.1177/00220345700490031501. PMID 5269110.
- Sasagawa I (June 1989). "The fine structure of initial mineralisation during tooth development in the gummy shark, Mustelus manazo, Elasmobranchia". Journal of Anatomy. 164: 175–87. PMC 1256608. PMID 2606790.
- Michael E. Williams, Jaws: The early years, found here .
- Luan, X.; Ito, Y.; Diekwisch, T.G.H. (2005). "Evolution and development of Hertwig's epithelial root sheath". Developmental Dynamics. 235 (5): 1167–1180. doi:10.1002/dvdy.20674. PMC 2734338. PMID 16450392.
- Martin, R. Aidan. "Skin of the Teeth". Retrieved 2007-08-28.
- Gilbertson, Lance (1999). Zoology Laboratory Manual. New York: McGraw-Hill Companies, Inc. ISBN 978-0-07-237716-3.
- Witten, P. E.; Hall, B. K. (2003). "Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning". Journal of Anatomy. 203 (5): 435–450. doi:10.1046/j.1469-7580.2003.00239.x. PMC 1571185. PMID 14635799.
- Groot, C.; Margolis, L. (1991). Pacific salmon life histories. UBC Press. p. 143. ISBN 978-0-7748-0359-5.
- Lee, H. J.; Kusche, H.; Meyer, A. (2012). "Handed Foraging Behavior in Scale-Eating Cichlid Fish: Its Potential Role in Shaping Morphological Asymmetry". PLOS ONE. 7 (9): e44670. doi:10.1371/journal.pone.0044670. PMC 3435272. PMID 22970282.
- Hori, M. (1993). "Frequency-dependent natural selection in the handedness of scale-eating cichlid fish". Science. 260 (5105): 216–219. doi:10.1126/science.260.5105.216. PMID 17807183. S2CID 33113282.
- Stewart, T. A.; Albertson, R. C. (2010). "Evolution of a unique predatory feeding apparatus: functional anatomy, development and a genetic locus for jaw laterality in Lake Tanganyika scale-eating cichlids". BMC Biology. 8 (1): 8. doi:10.1186/1741-7007-8-8. PMC 2828976. PMID 20102595.
- Moritz Muschick, Adrian Indermaur, Walter Salzburger : Convergent Evolution within Adaptive Radiation of Cichlid Fishes . In: Current Biology . Vol. 22 , No. 24 , 18 December 2012, ISSN 0960-9822 , pp. 2362-2368 , doi : 10.1016 / j.cub.2012.10.048 , PMID 23159601 ( cell.com [accessed April 7, 2016])
- Jorge R. Casciotta, Gloria Arratia: Jaws and teeth of American cichlids (Pisces: Labroidei) . In: Journal of Morphology . Vol. 217 , No. 1 , 1 July 1993, ISSN 1097-4687 , pp. 1-36 , doi : 10.1002 / jmor.1052170102
- Edward D. Burress: Cichlid fishes as models of ecological diversification: patterns, mechanisms, and consequences . In: Hydrobiologia . Vol. 748 , No. 1 , 18 July 2014, ISSN 0018-8158 , pp. 7-27 , doi : 10.1007 / s10750-014-1960-z
- Edward D. Burress, Alejandro Duarte, Michael M. Gangloff, Lynn Siefferman: Isotopic trophic guild structure of a diverse subtropical South American fish community . In: Ecology of Freshwater Fish . Vol. 22 , No. 1 , Jan. 1, 2013, ISSN 1600-0633 , pp. 66-72 , doi : 10.1111 / eff.12002 .
- Martin J. Genner, George F. Turner, Stephen J. Hawkins: Foraging of Rocky Habitat Cichlid Fishes in Lake Malawi: Coexistence through Niche Partitioning? In: Oecologia . Vol. 121 , No. 2 , January 1, 1999, pp. 283-292 , JSTOR : 4222466 .
- Kenaley, C. P. (2007). "Revision of the Stoplight Loosejaw Genus Malacosteus (Teleostei: Stomiidae: Malacosteinae), with Description of a New Species from the Temperate Southern Hemisphere and Indian Ocean". Copeia. 2007 (4): 886–900. doi:10.1643/0045-8511(2007)7[886:ROTSLG]2.0.CO;2.
- Sutton, T. T. (November 2005). "Trophic ecology of the deep-sea fish Malacosteus niger (Pisces: Stomiidae): An enigmatic feeding ecology to facilitate a unique visual system?". Deep-Sea Research Part I: Oceanographic Research Papers. 52 (11): 2065–2076. doi:10.1016/j.dsr.2005.06.011.
- Nelson, Joseph, S. (2006). Fishes of the World. John Wiley & Sons, Inc. ISBN 978-0-471-25031-9.
- Benton 2005.
- Kimmel, C. B.; Miller, C. T.; Keynes, R. J. (2001). "Neural crest patterning and the evolution of the jaw". Journal of Anatomy. 199 (1&2): 105–119. doi:10.1017/S0021878201008068. PMC 1594948. PMID 11523812.
- Janvier, P. (2007). "Homologies and Evolutionary Transitions in Early Vertebrate History". In Anderson, J. S.; Sues, H.-D. (eds.). Major Transitions in Vertebrate Evolution. Indiana University Press. pp. 57–121. ISBN 978-0-253-34926-2.
- Khonsari, R. H.; Li, B.; Vernier, P.; Northcutt, R. G.; Janvier, P. (2009). "Agnathan brain anatomy and craniate phylogeny". Acta Zoologica. 90 (s1): 52–68. doi:10.1111/j.1463-6395.2008.00388.x. S2CID 56425436.
- For example: (1) both sets of bones are made from neural crest cells (rather than mesodermal tissue like most other bones); (2) both structures form the upper and lower bars that bend forward and are hinged in the middle; and (3) the musculature of the jaw seem homologous to the gill arches of jawless fishes. (Gilbert 2000)
- Gilbert (2000). Evolutionary Embryology.
- Clack, J. A. (1994). "Earliest known tetrapod braincase and the evolution of the stapes and fenestra ovalis". Nature. 369 (6479): 392–394. doi:10.1038/369392a0.
- Donoghue, P. C.; Purnell, M. A. (2005). "Genome duplication, extinction and vertebrate evolution". Trends in Ecology & Evolution. 20 (6): 312–319. doi:10.1016/j.tree.2005.04.008. PMID 16701387.
- Forey, P. L.; Janvier, P. (1993). "Agnathans and the origin of jawed vertebrates". Nature. 361 (6408): 129–134. doi:10.1038/361129a0.
- "Placodermi: Overview". Palaeos. Retrieved 10 December 2014.
- "Acanthodii". Palaeos. Retrieved 10 December 2014.
- "More About Placoderms". Devonian Times. 9 July 2005.
- "Ancient Fish With Killer Bite". Science News. May 19, 2009.
- Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 33. ISBN 978-1-84028-152-1.
- "Monster fish crushed opposition with strongest bite ever". The Sydney Morning Herald. November 30, 2006.
- Anderson, P.S.L.; Westneat, M. (2007). "Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator". Biology Letters. 3 (1): 76–79. doi:10.1098/rsbl.2006.0569. PMC 2373817. PMID 17443970.
- Anderson, P. S. L.; Westneat, M. (2009). "A biomechanical model of feeding kinematics for Dunkleosteus terrelli (Arthrodira, Placodermi)". Paleobiology. 35 (2): 251–269. doi:10.1666/08011.1.
- "More About Acanthodians (spiny fins)". Devonian Times. 9 July 2005.
- Smith, M.M.; Coates, M.I. (2000). "10. Evolutionary origins of teeth and jaws: developmental models and phylogenetic patterns". In Teaford, Mark F.; Smith, Moya Meredith; Ferguson, Mark W.J. (eds.). Development, function and evolution of teeth. Cambridge: Cambridge University Press. p. 145. ISBN 978-0-521-57011-4.
- Britt, Robert Roy (28 November 2006). "Prehistoric Fish Had Most Powerful Jaws". Live Science.
- "The Gill Arches: Meckel's Cartilage". palaeos. Retrieved 4 December 2014.
Other reading
- Benton, Michael J (2009). Vertebrate Palaeontology (3rd ed.). John Wiley & Sons. ISBN 978-1-4051-4449-0.
- Botella, H.; Blom, H.; Dorka, M.; Ahlberg, P. E.; Janvier, P. (2007). "Jaws and teeth of the earliest bony fishes". Nature. 448 (7153): 583–586. doi:10.1038/nature05989. PMID 17671501.
- Compagnucci, C; Debiais-Thibaud, M; Coolen, M; Fish, J; Griffin, J N; Bertocchini, F; Minoux, M; Rijli, F M; Borday-Birraux, V; Casane, D; Mazanc, S; Depew, M J (2013). "Pattern and polarity in the development and evolution of the gnathostome jaw: Both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula". Developmental Biology. 377 (2): 428–448. doi:10.1016/j.ydbio.2013.02.022. PMID 23473983.
- Depew, M J; Lufkin, T; Rubenstein, J L (2002). "Specification of jaw subdivisions by Dlx genes". Science. 298 (5592): 381–385. doi:10.1126/science.1075703. PMID 12193642.
- Forey, Peter; Janvier, Philippe (2000). "Agnathans and the origin of jawed vertebrates". In Gee, Henry (ed.). Shaking the tree: readings from Nature in the history of life. USA: University of Chicago Press; Nature/Macmillan Magazines. pp. 251–266. ISBN 978-0-226-28497-2.
- Gilbert, Scott F. (2000). "The anatomical tradition: Evolutionary Embryology: Embryonic homologies". Developmental Biology. Sunderland (MA): Sinauer Associates, Inc. (NCBI). Retrieved 2018-04-09. (3rd and 4th paras, One of the most celebrated cases...)
- Hulsey, CD; Fraser, GJ; Streelman, JT (2005). "Evolution and development of complex biomechanical systems: 300 million years of fish jaws". Zebrafish. 2 (4): 243–257. CiteSeerX 10.1.1.210.7203. doi:10.1089/zeb.2005.2.243. PMID 18248183.
- Koentges, G; Matsuoka, T (2002). "Jaws of the fates". Science. 298 (5592): 371–373. doi:10.1126/science.1077706. PMID 12376690.
- Lingham-Soliar, T. (2014). "The First Vertebrates, Jawless Fishes, the Agnathans" (PDF). The Vertebrate Integument. 1. Springer. pp. 11–31. ISBN 978-3-642-53748-6.
- Lingham-Soliar, T. (2014). "The Earliest Jawed Vertebrates, the Gnathostomes". The Vertebrate Integument. 1. Springer. pp. 33–58. ISBN 978-3-642-53748-6.
- Mallatt, J. (2008). "The origin of the vertebrate jaw: Neoclassical ideas versus newer, development-based ideas". Zoological Science. 25 (10): 990–998. doi:10.2108/zsj.25.990. PMID 19267635.
- Mehta, R. S.; Wainwright, P. C. (2008). "Functional morphology of the pharyngeal jaw apparatus in moray eels" (PDF). Journal of Morphology. 269 (5): 604–619. doi:10.1002/jmor.10612. PMID 18196573. Archived from the original (PDF) on 2014-12-09. Retrieved 2014-12-09.
- Muschick, M.; Salzburger, W. (2013). "Pharyngeal jaws and their evolutionary, ecological and behavioural significance" (PDF). In Muschick, Moritz (ed.). Convergence and plasticity in the adaptive radiation of cichlid fishes (PhD thesis). University of Basel. pp. 13–37.
- Oisi, Y; Ota, K G; Kuraku, S; Fujimoto, S; Kuratani, S (2013). "Craniofacial development of hagfishes and the evolution of vertebrates". Nature. 493 (7431): 175–180. doi:10.1038/nature11794. PMID 23254938.
- Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 173–177. ISBN 978-0-03-910284-5.
- Soukup, V; Horácek, I; Cerny, R (2013). "Development and evolution of the vertebrate primary mouth". Journal of Anatomy. 222 (1): 79–99. doi:10.1111/j.1469-7580.2012.01540.x. PMC 3552417. PMID 22804777.
- Wainwright, P. C. (2006). "Functional Morphology of the Pharyngeal Jaw Apparatus". In Shadwick, R. E.; Lauder, G. V. (eds.). Fish Biomechanics. Fish Physiology. 23. Academic Press. pp. 77–102. ISBN 978-0-08-047776-3. Full view
- Westneat, M. W. (2006). "Skull Biomechanics and Suction Feeding in Fishes". In Shadwick, R. E.; Lauder, G. V. (eds.). Fish Biomechanics. Fish Physiology. 23. Academic Press. pp. 29–76. ISBN 978-0-08-047776-3.
- Westneat, Mark W. (2004). "Evolution of levers and linkages in the feeding mechanisms of fishes". Integrative and Comparative Biology. 44 (5): 378–389. doi:10.1093/icb/44.5.378. PMID 21676723.
External links
| External video | |
|---|---|
- "Moray Eels Are Uniquely Equipped to Pack Big Prey Into Their Narrow Bodies" (Press release). National Science Foundation. September 5, 2007.
- Myers, PZ (13 March 2007). "Evolution of the jaw". Pharyngula.
- Barford, Eliot (25 September 2013). "Ancient fish face shows roots of modern jaw". News. Nature.
- Zhu, Min; Yu, Xiaobo; Erik Ahlberg, Per; Choo, Brian; Lu, Jing; Qiao, Tuo; Qu, Qingming; Zhao, Wenjin; Jia, Liantao; Blom, Henning; Zhu, You’an (2013). "A Silurian placoderm with osteichthyan-like marginal jaw bones". Nature. 502 (7470): 188–193. doi:10.1038/nature12617. PMID 24067611.
